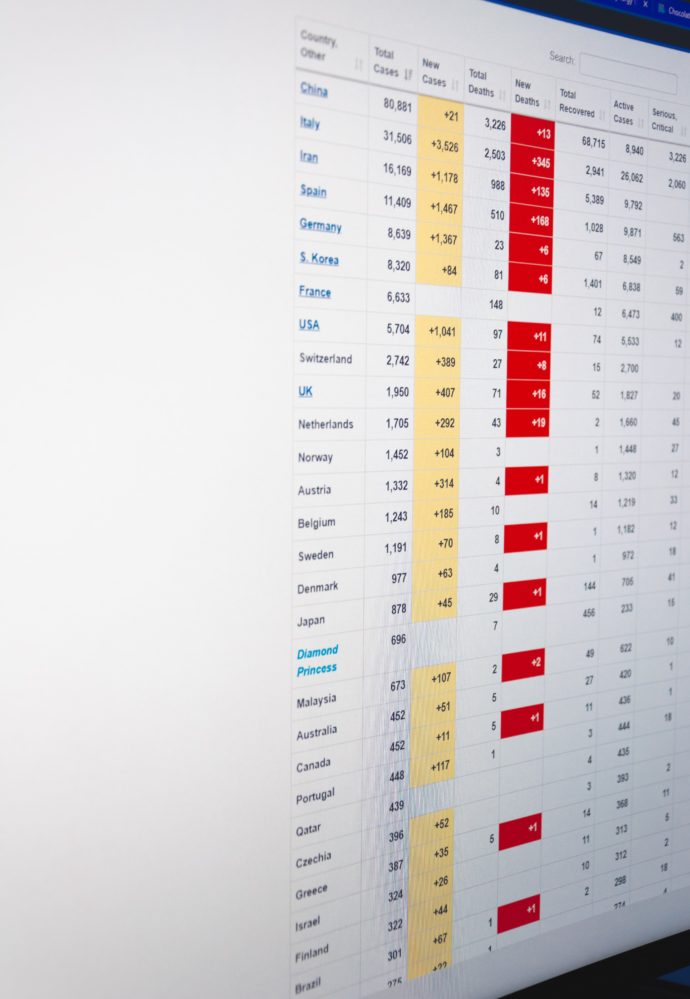
Because Congress is out of town until after Labor Day. let’s lead with the story that caused the FEHBlog to levitate out of his easy chair yesterday. The Yale News headline says it all — “Quick and affordable saliva-based COVID-19 test developed by Yale scientists receives FDA Emergency Use Authorization.”
A couple weeks ago the FEHBlog mentioned a USA Today story about an Xprize competition to find a quick and inexpensive COVID-19 test that would facilitate going back to work, school, etc. The Xprize competition’s first round closes next week and we already have one winner.
Check this out:
Wide-spread testing is critical for our control efforts. We simplified the test so that it only costs a couple of dollars for reagents, and we expect that labs will only charge about $10 per sample. If cheap alternatives like SalivaDirect can be implemented across the country, we may finally get a handle on this pandemic, even before a vaccine,” said [Yale Assistant Professor of Public Health Nathan] Grubaugh.
One of the team’s goals was to eliminate the expensive saliva collection tubes that other companies use to preserve the virus for detection. In a separate study led by Wyllie and the team at the Yale School of Public Health, and recently published on medRxiv, they found that SARS-CoV-2 is stable in saliva for prolonged periods at warm temperatures, and that preservatives or specialized tubes are not necessary for collection of saliva.
The Yale researchers validated their test with the cooperation of the National Basketball Association. It was interesting to watch this season’s opening Hard Knocks show on HBO which showed how NFL teams similarly are focused both on the team’s schedule and COVID-19.
In litigation news —
- Last Friday, as Healthcare Dive reports, the U.S. Court of Appeals for the Federal Circuit handed health insurers participating in the ACA’s marketplace another win against the federal government. This time the unpaid amounts involve reimbursable cost sharing reductions for low income marketplace participants.
- On Tuesday, August 18, the Health and Human Services Department’s revised rule on the ACA’s individual non-discrimination provision, Section 1557, takes effect. The federal government, as requested by the Court, filed a sur-reply on the standing issue last Monday, and one of the plaintiff’s advised the Court on Wednesday that the U.S. Court of Appeals for the Fifth Circuit has revived the dormant Franciscan Alliance case which is the granddaddy of challenges to the 1557 rule (although it was filed in an effort to challenge the Obama Administration’s versions of the rule.)
In other news, Health Payer Intelligence reports that
Payers can play a significant role in decreasing the expense and complexity of serious illness care for patients through whole person care and palliative care, a study from America’s Health Insurance Plans (AHIP) found.
“Recognizing the difficulties of serious illness, health insurance providers have set out to help, support, and ease the journey for patients, caregivers and loved ones,” the AHIP study stated.
“Ensuring access to tools, education, and services for patients and their loved ones during a difficult time can provide the opportunity to plan, allow patients to maintain their dignity and choice, and support loved ones to know their role and how best they can help.”
Check it out.
Finally, the Federal News Network informs us that the General Services Administration made no changes to the current standard per diem rates for hotels, meals, and incidental expenses of federal employee business travel within the contiguous United States effective for the next federal fiscal year beginning October 1, 2020. What’s more,
There are, however 319 non-standard areas (NSAs), which have higher per diem rates than the standard CONUS allowance. GSA added one area, Albuquerque, New Mexico, as a new NSA location this year. Four locations came off the NSA list from 2020 and will now receive the standard per diem rate: Gainesville, FL; Atlantic City, NJ; College Station, NJ, and Abingdon, VA.
These rates also apply to government contracts such as experience rated FEHB carriers that are subject to the Federal Acquisition Regulation’s Cost Principle. The GSA assumes, of course, that business travel will resume next year, and in view of the low cost saliva test, it just might.