Tuesday’s Tidbits

Govexec reports that “the Senate voted 81-13 on Tuesday to confirm Jason Miller to be deputy director for management for the Office of Management and Budget. Miller is a former Obama administration economic adviser and most recently a nonresident senior fellow at the Brookings Institution and CEO of the Greater Washington Partnership, a nonprofit civic alliance.”
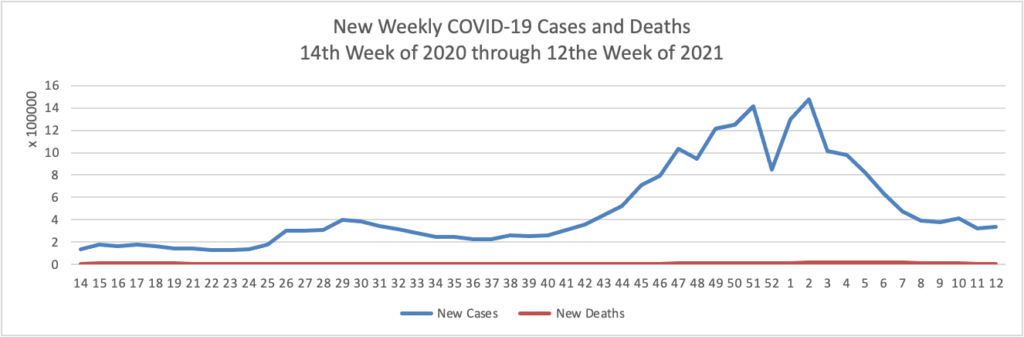
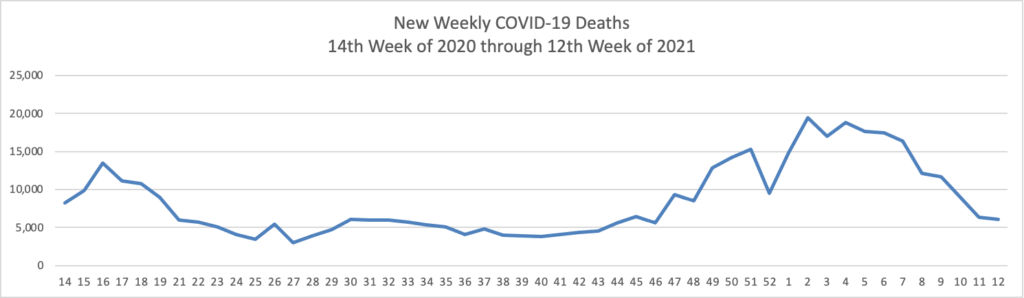
Julie Appleby of Kaiser Health News writes on the gradual rollback of COVID-19 treatment coverage with no member cost sharing in 2021.
Anthem, for example, stopped them at the end of January. UnitedHealth, another of the nation’s largest insurers, began rolling back waivers in the fall, finishing up by the end of March. Deductible-free inpatient treatment for covid through Aetna expired Feb. 28.
A few insurers continue to forgo patient cost-sharing in some types of policies. Humana, for example, has left the cost-sharing waiver in place for Medicare Advantage members, but dropped it on Jan. 1 for those in job-based group plans.
Not all are making the changes.
For example, Premera Blue Cross in Washington and Sharp Health Plan in California have extended treatment cost waivers through June. Kaiser Permanente said it is keeping its program in place for members diagnosed with covid and has not set an end date. Meanwhile, UPMC in Pittsburgh planned to continue to waive all copayments and deductibles for in-network treatment through April 20.
Healthcare Dive reports
U.S. hospitals continue to struggle under the ongoing weight of the pandemic and its financial pressure, reporting a mixed performance in March, according to a new report from Kaufman Hall.
Volumes continued to decline, while revenues and expenses generally rose compared to the same time last year. Margins increased on both a year-to-date and year-over-year basis, but that’s largely due to measuring performance this year with last March, when hospitals were hit hard by the effects of state lockdowns and a pause in non-essential procedures, the consultancy said.
Researchers expect continued margin and revenue gains in the next few months, especially in comparison to record-poor performance in the first few months last year. Some gains are due to returning patient volumes, but the report warns the impacts of COVID-19 on providers are far from over.
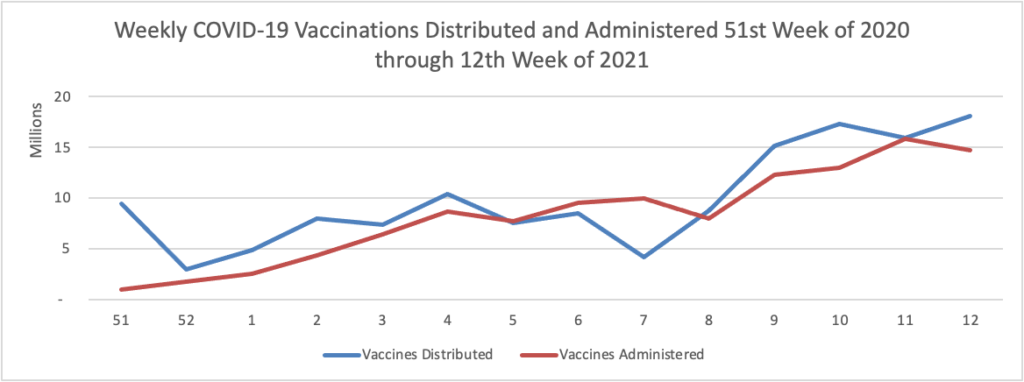
Here is a link to today’s Centers for Disease Control’s “Interim Public Health Recommendations for Fully Vaccinated People.” The AP reports that “Some experts portrayed the relaxed guidance as a reward and a motivator for more people to get vaccinated — a message President Joe Biden sounded, too.” The FEHBlog honestly see the new guidance as too complicated and he will maintain his current mask wearing practices for a couple more months.
From the government contracting front
- Here is a link to the President’s executive order raising the minimum wage on federal contracts for services and construction to $15 per hour. FEHB contracts do not fall into these classifications in the FEHBlog’s opinion.
- The Equal Employment Opportunity Commission is revving up the EEO-1 reporting process. “The EEO-1 Component 1 report is a mandatory annual data collection that requires all private sector employers with 100 or more employees, and federal contractors with 50 or more employees meeting certain criteria, to submit demographic workforce data, including data by race/ethnicity, sex and job categories. * * * After delaying the opening of the 2019 EEO-1 Component 1 data collection because of the COVID-19 public health emergency, the EEOC has announced that the 2019 and 2020 EEO-1 Component 1 data collection is NOW OPEN. Eligible employers have until Monday, July 19, 2021 to submit two years of data.”