Weekend update

The Senate remains on a State work break this week while the House of Representatives has scheduled a few Committee meetings but no floor voting.
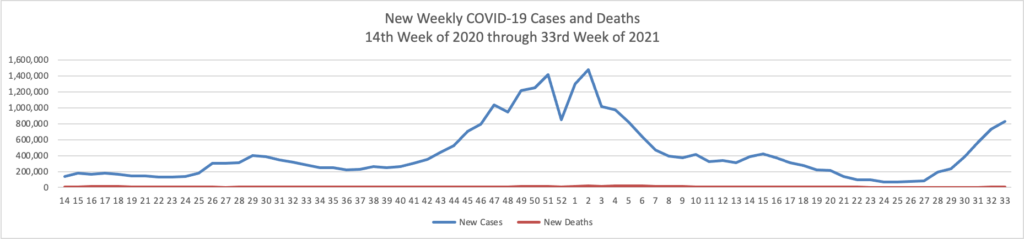
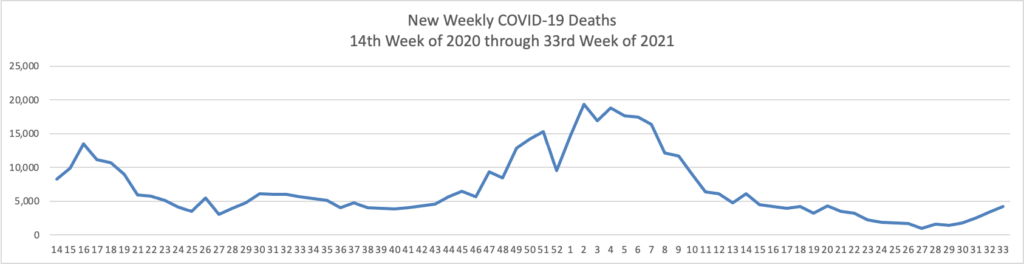
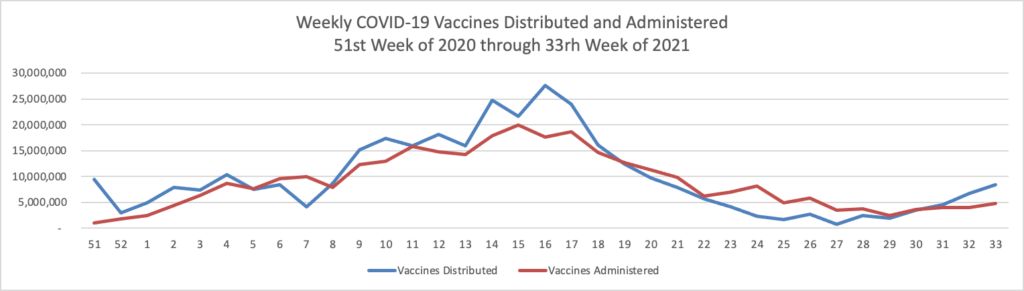
The CDC’s Advisory Committee on Immunization Practices meets tomorrow and Tuesday to discuss expanding Pfizer/Biontech boosters to the age 16 and older population. Currently the boosters are only available for the moderately and severely immunocompromised population. (N.B. In Monday’s New York Times David Leonhardt writes an insightful column on the expansion question. He suggests that COVID-19 vaccine immunity may not be waning enough to justify the expansion.
Immunity does probably wane modestly within the first year of receiving a shot. For this reason, booster shots make sense for vulnerable people, many experts believe. As Dr. Céline Gounder of Bellevue Hospital Center told my colleague Apoorva Mandavilli, the C.D.C.’s data “support giving additional doses of vaccine to highly immunocompromised persons and nursing home residents, not to the general public.”
The current booster shots may do little good for most people. The vaccines continue to provide excellent protection against illness (as opposed to merely a positive Covid test). People will eventually need boosters, but it may make more sense to wait for one specifically designed to combat a variant. “We don’t know whether a non-Delta booster would improve protection against Delta,” Dr. Aaron Richterman of the University of Pennsylvania told me.
A national policy of frequent booster shots has significant costs, financially and otherwise. Among other things, the exaggerated discussion of waning immunity contributes to vaccine skepticism.
While Americans are focusing on booster shots, other policies may do much more to beat back Covid, including more vaccine mandates in the U.S.; a more rapid push to vaccinate the world(and prevent other variants from taking root); and an accelerated F.D.A. study of vaccines for children.
As always, we should be open to changing our minds as we get new evidence. As Richterman puts it, “We have time to gather the appropriate evidence before rushing into boosters.”
FCW interviewed “OPM’s Senior Advisor to the Director on Diversity, Equity, Inclusion and Accessibility Mini Timmaraju.” The President’s recent executive order on this topic set Oct. 3 as the deadline for agencies to finish a preliminary assessment of human resources practices and agency workforces.” Ms. Timmaraju’s team is helping other agencies prepare these documents. The article further explains that
At the same time, OPM is working on its own reorganization, which will reestablish an Office of Diversity, Equity, Inclusion and Accessibility as a standalone program office that reports directly to the OPM director.
It was absorbed into the Employee Services Center during the Trump administration, and its workforce has been “reduced” over the last four years, Timmaraju said.
Internal DEIA work at OPM is also moving from the agency’s Equal Opportunity Employment to Human Resources.
The new office will focus on governmentwide initiatives, according to OPM. It’ll have three sub-organizations, focused on policy and development, analytics and accountability and outreach and technical assistance.
The goal is to rightsize the team back to similar levels seen during the Obama administration, Timmaraju said. The office had 12 full-time equivalent employees in fiscal year 2016, according to budget documents.
OPM is also recruiting a Senior Executive Service-level director for the office. The job listing on USAJobs says that the agency is currently reviewing applications.
In healthcare news
- The Wall Street Journal reports that “CVS Health is among several retailers including Walmart Inc. and Walgreens Boots Alliance Inc.,that are experimenting with offering counseling services in or near stores. They see potential as the Covid-19 pandemic has prompted more people to seek help for addiction, depression and other issues, according to federal data. “It’s creative and we certainly need the help,” said Ken Duckworth, medical director of the National Alliance on Mental Illness. “It’s an interesting idea to post a mental-health resource at a place where people already are at.” The FEHBlog heartily agrees.
- The Food and Drug Administration approved on Friday August 27 “the MicroTransponder Vivistim Paired VNS System (Vivistim System), a first-of-its-kind, drug-free rehabilitation system intended to treat moderate to severe upper extremity motor deficits associated with chronic ischemic stroke—a stroke caused by a blockage of blood flow to the brain with long-lasting symptoms—using vagus nerve stimulation (VNS). ‘People who have lost mobility in their hands and arms due to ischemic stroke are often limited in their treatment options for regaining motor function” said Christopher M. Loftus, M.D., acting director of the FDA’s Center for Devices and Radiological Health’s Office of Neurological and Physical Medicine Devices. “Today’s approval of the Vivistim Paired VNS System offers the first stroke rehabilitation option using vagus nerve stimulation. Used alongside rehabilitative exercise, this device may offer benefit to those who have lost function in their upper limbs due to ischemic stroke.’” Cool.
- The Drug Enforcement Administration offers ten strategies for keep youngsters and teenagers off drugs. A friend of the FEHBlog, who is a psychiatrist explained that brain development continues until the mid-twenties. If you start a bad habit before your late twenties it will be harder to break than a bad habit that begins after the mid-twenties. Business Insider sums it up as follows: “Neuroscientists are confirming what car rental places already figured out — the brain doesn’t fully mature until age 25. Up until this age, the prefrontal cortex — the part of the brain that helps curb impulsive behavior — is not yet fully developed.”