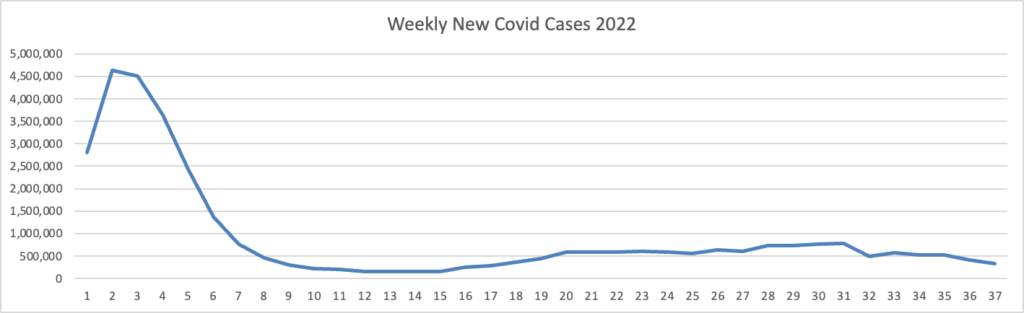
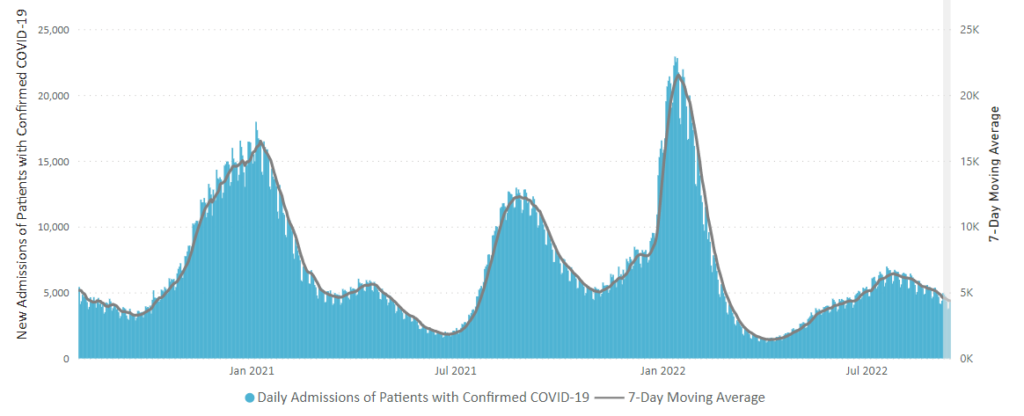
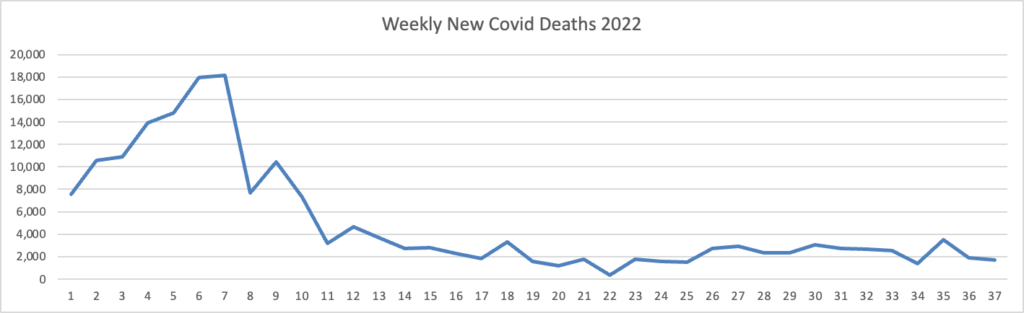
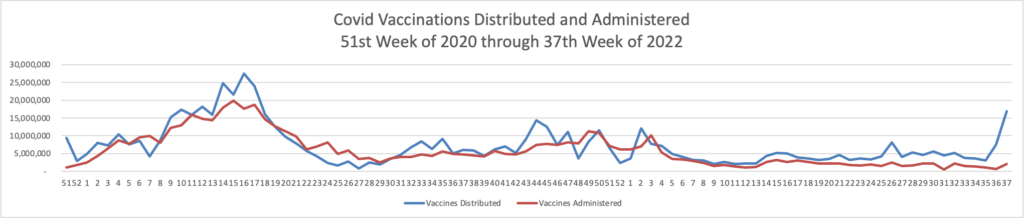
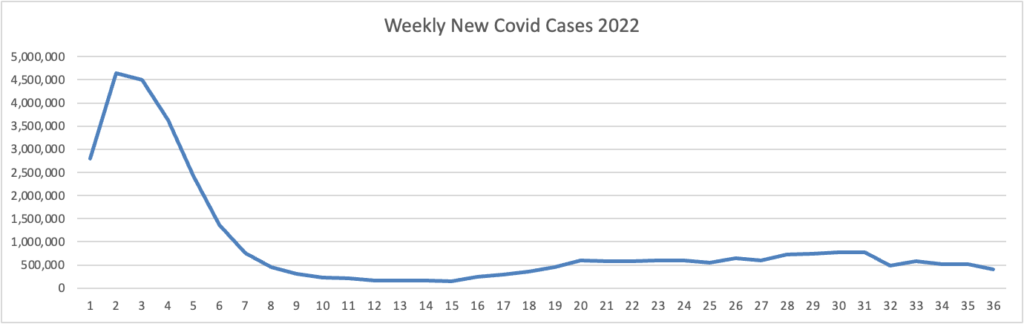
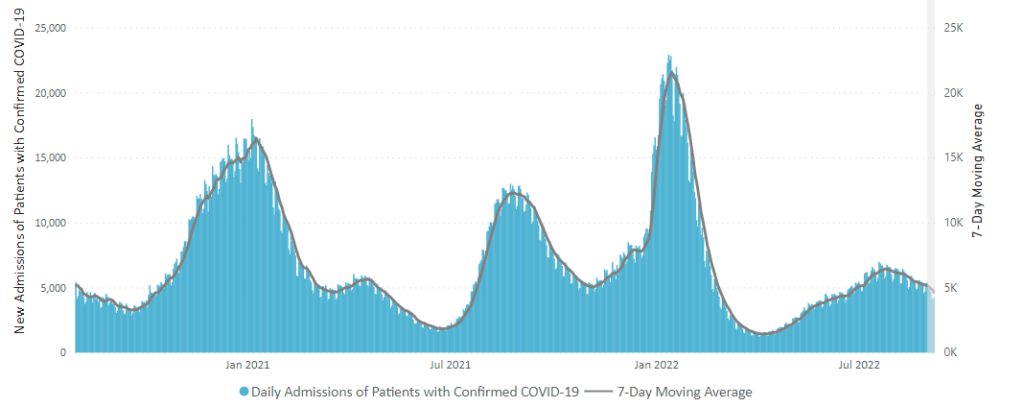
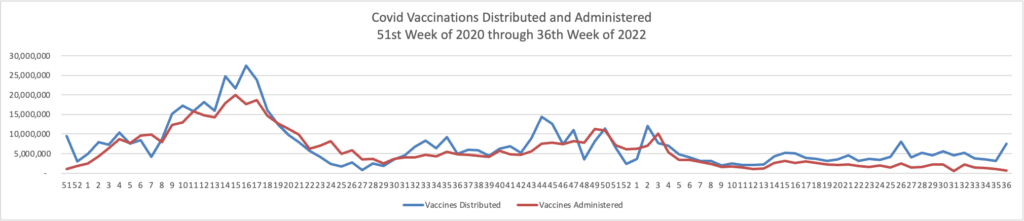
Tuesday Tidbits

From Capitol Hill, Govexec lays out what appears to be an unnecessarily complicated path to a continuing resolution funding the federal government for 10 weeks into the new federal fiscal year beginning October 1. The Senate majority leadership crafted the rocky path that stems from the compromise which lead to Congressional passage of the budget reconciliation act earlier this summer.
From the No Surprises Act front, the American Medical Association informs us
The AHA and American Medical Association today moved to dismiss their challenge to the federal government’s September 2021 interim final rule governing the No Surprises Act’s independent dispute resolution process.
The groups challenged the rule in a District of Columbia court last December, but the lawsuit became moot when the Administration released a revised final rule on Aug. 26. However, the AHA and AMA remain concerned that the final rule continues to favor insurers and does not line up with what Congress intended when it passed the law.
In a joint statement the AHA and AMA said, “No patient should fear receiving a surprise medical bill. That is why the AHA and AMA strongly supported the No Surprises Act to protect patients from unexpected medical bills and keep them out of the middle of any billing disputes between providers and commercial health insurance companies. Congress enacted the law with a balanced, patient-friendly approach, and it should be implemented that way. We have serious concerns that the August 2022 final rule departs from Congressional intent just as the September 2021 interim final rule did. Hospitals and doctors intend to make our voices heard in the courts very soon about these continued problems.”
The AHA and AMA’s suit did not seek to prevent the law’s core patient protections from moving forward. It sought only to force the Administration to bring the regulations in line with the law before the dispute negotiations begin.
The AHA / AMA lawsuit is consolidated with a suit filed by an air ambulance association which may explain why these two large provider associations are dismissing its case rather than amending their complaint. The FEHBlog does not understand why the provider associations refuse to give the new rule a chance before bringing another expensive lawsuit.
From the U.S. healthcare business front —
Fierce Healthcare reports
Walgreens Boots Alliance on Tuesday said it will buy the remaining stake in specialty pharmacy company Shields Health Solutions for approximately $1.37 billion.
Walgreens last year spent $970 million to increase its stake in the company to 71%, according to Reuters, with the possibility of taking full ownership over the pharmacy company.
The transaction is expected to be completed by the end of the year. * * *
As a specialty pharmacy, Shields offers medications with unique handling, administration and monitoring requirements. Specialty drugs are used to treat complex or rare conditions such as cancer, hepatitis and transplants. Shields currently names 30 health systems as partners, including 1,000 hospitals.
Employer health startup Transcarent is making its next move with the launch of its new pharmacy program.
Transcarent’s Pharmacy Care offering is designed to be fully transparent and integrate with its other platforms. The goal, executives said, is to break through the noise for members and make it easier for them to understand their pharmacy benefits while offering employers full control over formulary, benefit design and data.
The platform is available to self-funded employers as well as health systems, Transcarent said in an announcement. Snezana Mahon, Transcarent’s chief operating officer, told Fierce Healthcare that the company’s employer clients have felt the market changes and are seeking a way to “coexist” in a world where there are traditional pharmacy benefits, cash pay and coupon cards all working together.
From the healthcare quality front, Beckers Hospital Review calls attention to
A new data visualizer shows the 10 most similar hospitals to any one benchmark hospital, challenging traditional, ordinal rank lists like those from U.S. News & World Report.
SimilarityIndex | Hospitals comes from Trilliant Health Labs, which created the tool so health economy stakeholders can learn how similar a selected benchmark hospital is to — or different from — highly regarded U.S. hospitals.
Users can compare hospitals to find peers in either quality alone or aggregate — the latter reflects an equally weighted combination of measurements in the categories of hospital quality (including 30-day mortality and readmission rates), outpatient service line, financial (including operating margin and average inpatient service costs), patient mix and market share.
Nifty.
From the public health front —
- Medscape tells us
The US Preventive Services Task Force (USPSTF) today posted for public comment draft recommendations on screening for anxiety, depression, and suicide risk in adults.
For the first time, the task force is recommending screening all adults aged 64 and younger for anxiety — including pregnant and postpartum women.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in this population has a moderate net benefit, the task force notes in a draft recommendation statement posted on its website.
The recommendation applies to adults aged 19-64 years who do not have a diagnosed mental health disorder or are not showing recognized signs or symptoms of anxiety.
The public comment deadline is October 17.
- The Wall Street Journal offers advice on timing the annual flu shot and the upcoming flu season in general.
- The CDC released a vital signs report warning that rates of screening and treatment of children with sickle cell anemia for life-threatening problems are far too low.
Two recommended healthcare measures to prevent complications in children with sickle cell anemia are:
* Transcranial doppler (TCD) ultrasound screening, which identifies children with increased risk for stroke.
* Hydroxyurea therapy, which reduces the occurrence of several complications, including severe acute pain episodes and acute chest syndrome, which can result in lung injury and trouble breathing.
Far too few patients are receiving these potentially lifesaving prevention measures, recommended by an expert panel in 2014.
- The CDC also called attention to its website about gestational diabetes.
From the Rx coverage front, Bio Pharma Dive relates
The Food and Drug Administration on Friday [September 16] granted accelerated approval to a personalized gene therapy for an ultra-rare childhood brain disease, called cerebral adrenoleukodystrophy or CALD.
Built from a patient’s own stem cells, the therapy is the first medicine to be made available in the U.S. for CALD, which affects young boys and typically results in severe disability or death. It was developed by the biotechnology company Bluebird bio and will be sold as Skysona.
Its approval is Bluebird’s second in four weeks, following an Aug. 17 FDA decision on another gene therapy from the company for the blood disorder beta thalassemia. * * *
In the U.S., an estimated 50 boys are born each year who will go on to develop CALD. Bluebird expects to treat about 10 annually.
Meant to be a one-time infusion, Skysona will cost $3 million. The price tag makes the therapy one of the most expensive ever launched on a single-use basis, exceeding the $2.8 million cost of Bluebird’s other gene therapy. * * *
Bluebird expects Skysona to be available by the end of the year, and is planning to work with a “limited number” of centers that are experienced in treating CALD and in stem cell transplantation, including Boston Children’s Hospital and CHOP [Children’s Hospital of Philadelphia].
[Due to the small number of patients, t]he company is not putting in place “outcomes-based” coverage agreements with insurers for Skysona as it did with its other gene therapy, for which it’s offering to reimburse part of the cost if patients don’t continue to benefit.
From the surveys department —
- Beckers Payer Issues reports
A majority of healthcare executives think value-based-care has replaced fee-for-service billing, a new survey found.
Of 160 C-suite executives and other high-level staff surveyed, just 4 percent said they think payers use traditional fee-for-service billing with no connection to quality and value. The majority of executives think payers use FFS models with connections to the quality and value of care taken into account.
The survey, conducted by business intelligence firm Morning Consult and health tech company Innovaccer, found just 1 percent of executives think FFS billing with no connection to value will be in use in 2025.
According to a Sept.14 news release, payers report that FFS billing with no account for value makes up more than 10 percent of billing, higher than providers estimated.
“So, providers think the transition to value has substantially occurred, when in fact we’re only at the very beginning,” Brian Silverstein, MD, Innovaccer’s chief population health officer, said in the release. “The amount of financial risk providers have is going to increase significantly in the next few years.”
- Beckers Hospital Review tells us “Patients who are publicly insured or uninsured are more likely to be treated unfairly in healthcare settings compared to patients with private insurance, according to a report from the Urban Institute with support from the Robert Wood Johnson Foundation.”
In closing Federal News Network shares the list of deserving federal employees receiving the 2022 Partnership for Public Service’s Samuel J. Heyman Service to America Medals — affectionately known as the Sammies. These awards “often dubbed the “Oscars” of federal service” will be presented at a gala tonight. Hearty congratulations to the award winners and the other nominees.