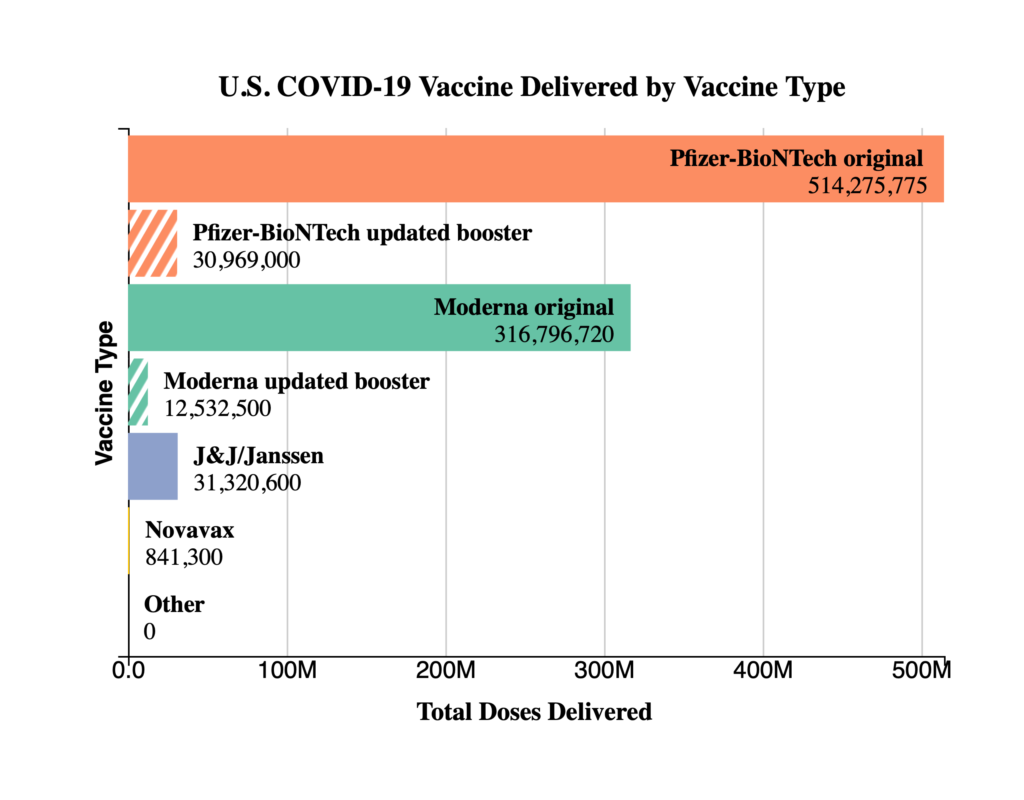
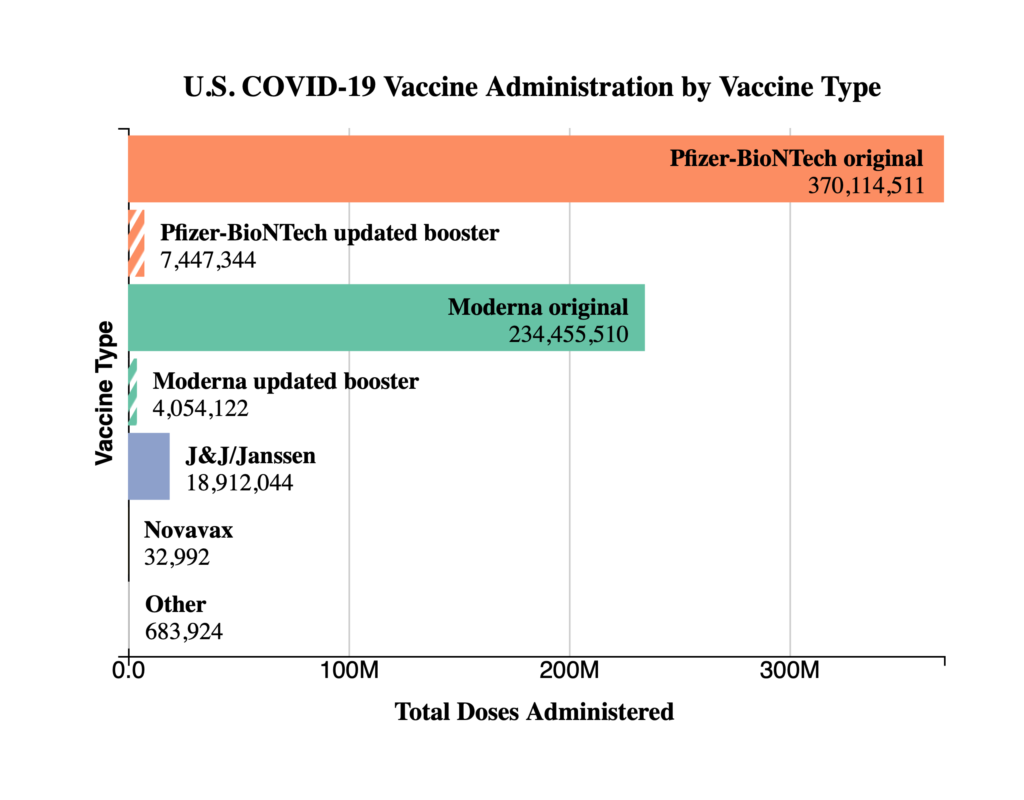
Thursday Miscellany

From the OPM front, an OPM press release informs us
The U.S. Office of Personnel Management (OPM) released government-wide results of the 2022 OPM FEVS today. The OPM FEVS is an employee survey that tracks how federal employees view their current work environment, including management, policies, and new initiatives. OPM FEVS is an unmatched government data asset that assists agencies to hire and support the skilled workforce needed to serve the American people.
According to Gallup, employee engagement for the total U.S. workforce has declined for the past two years by a total of four percentage points, the first time it has dropped in over a decade. The OPM FEVS government-wide employee engagement index dropped one percentage point from 2020 to 2021, and then stabilized above pre-pandemic levels at 71 percent in 2022. In 2019, this metric stood at 68 percent.
Additional highlights from the 2022 OPM FEVS government-wide results include:
* The Performance Confidence Index, which measures employees’ view that their work unit can achieve goals and produce at a high level, remains high at 84 percent.
* The 2022 OPM FEVS includes a new Diversity, Equity, Inclusion and Accessibility (DEIA) Index, which shows 69 percent of respondents report positive perceptions of agency practices related to DEIA.
* The 2022 OPM FEVS newly evaluates Innovation and to what extent leadership encourages and supports new ideas and innovative approaches. The survey scores show success and opportunities for innovation encouragement, with 64 percent of employees consistently looking for new ways to improve work and 56 percent noting that management encourages innovation.
In other encouraging news, Federal News Network reports
Suicides across the active duty U.S. military decreased over the past 18 months, driven by sharp drops in the Air Force and Marine Corps last year and a similar decline among Army soldiers during the first six months of this year, according to a new Pentagon report and preliminary data for 2022.
The numbers show a dramatic reversal of what has been a fairly steady increase in recent years.
The shift follows increased attention by senior military leaders and an array of new programs aimed at addressing what has been a persistent problem in all the services, although it’s unclear what impact any of the programs had or if pandemic-related restrictions played any role in the decline.
On a related note —
- The actuarial consulting firm WTW released the employer survey findings
Two out of three U.S. employers (67%) plan to make employee mental health and emotional wellbeing programs and solutions one of their top three health priorities over the next three years. Additionally, the number of employers that intend to offer designated mental health days could triple from 9% currently to 30% in the next two years.
- The U.S. Surgeon General offers best practices for designing employer-sponsored mental health programs.
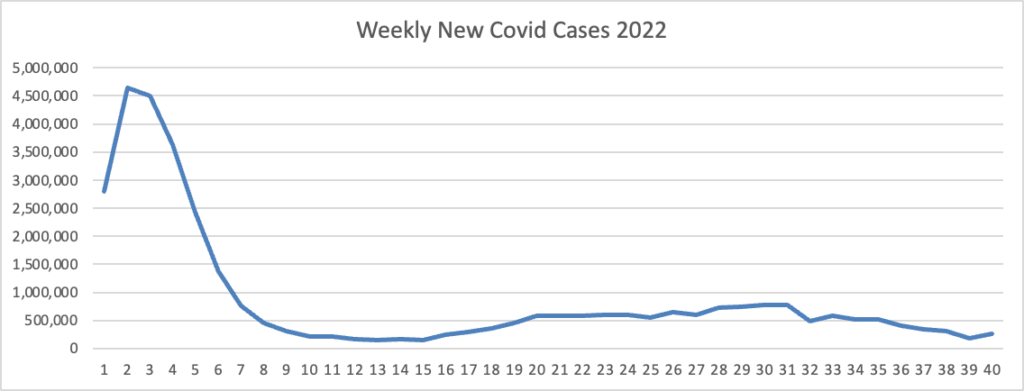
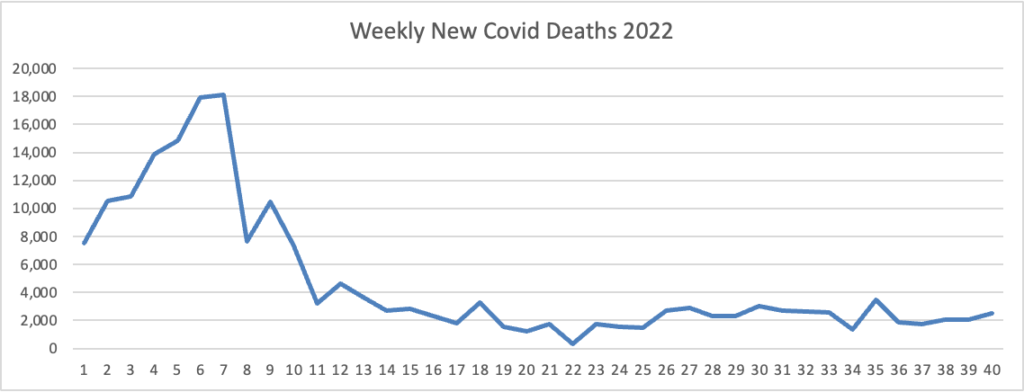
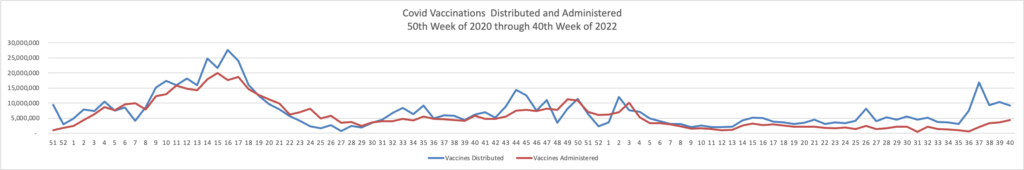
From the Omicron and siblings, front MedPage Today tells us
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted unanimously Thursday to add COVID-19 vaccination to its panel of routine immunizations for both kids and adults. The 15-0 vote does not mandate vaccination for children or adults or prevent unvaccinated children from attending school; it’s simply an annual update to the child and adult immunization schedules, panelists pointed out.
The ACIP decision does mandate that health plans cover Covid vaccines without member cost sharing after the public health emergency expires, likely next year.
In other public health news, the Wall Street Journal reports
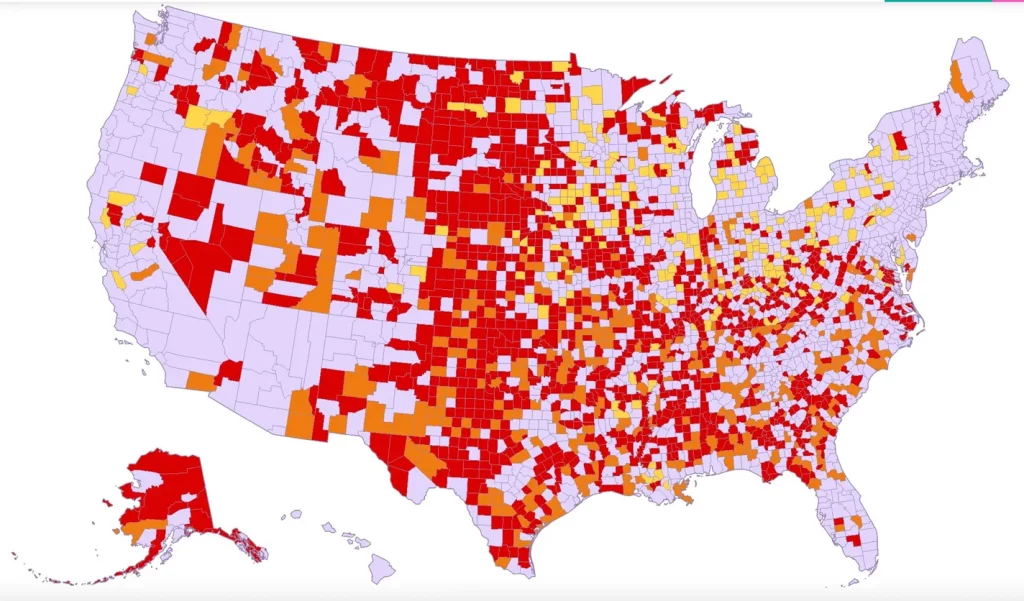
Physicians are reporting unseasonably high numbers of respiratory illnesses in children, straining many children’s hospitals before the typically busier winter months.
Juan Salazar, physician in chief at Connecticut Children’s Medical Center in Hartford, Conn., said a sharp increase in cases of respiratory syncytial virus, or RSV, has filled up hospital beds at his facility, creating capacity issues.
RSV is an easily transmissible virus that infects the respiratory tract. The virus spreads through droplets from coughing and sneezing and on surfaces. Positive tests for RSV have been on the rise across the U.S., according to the Centers for Disease Control and Prevention. The rise in cases has come ahead of the typical winter peak for such illnesses, hospital officials said.
For most people, RSV amounts to a cold, and nearly all children come in contact with the virus by the age of two, health authorities said. But it can be severe for some infants and older adults, especially for those that have pre-existing health conditions.
Much like influenza, RSV cases were flattened during the first year of the Covid-19 pandemic. The respiratory virus that typically circulates in the fall and winter then rebounded in the summer of 2021.
Is RSV the official name for the common cold? Calling Dr. Google. Perhaps people should choose to wear N-95 masks in the winter.
From the Rx coverage front
The Institute for Clinical and Economic Review (ICER) today released a Final Evidence Report assessing the comparative clinical effectiveness and value of subcutaneous semaglutide (Wegovy, Novo Nordisk), liraglutide (Saxenda, Novo Nordisk), phentermine/topiramate (Qsymia, Vivus Pharmaceuticals), and bupropion/naltrexone (Contrave, Currax Pharma) for the treatment of obesity.
“The vast majority of people with obesity cannot achieve sustained weight loss through diet and exercise alone,” said David Rind, MD, ICER’s Chief Medical Officer. “As such, obesity, and its resulting physical health, mental health, and social burdens is not a choice or failing, but a medical condition. The development of safe and effective medications for the treatment of obesity has long been a goal of medical research that now appears to be coming to fruition. With a condition affecting more than 40% of adults in the US, the focus should be on assuring that these medications are priced in alignment with their benefits so that they are accessible and affordable across US society.”
Downloads: Final Evidence Report | Report-at-a-Glance | Policy Recommendations
This report is worth a gander because OPM is requiring coverage of next-gen obesity drugs for 2023.
It turns out that October is health literacy month.
- The Labor Department’s Assistant Secretary for Employee Benefits offers employees five tips for making health benefits work.
- The HHS Agency for Healthcare Quality and Research gives healthcare providers a complete literacy manual, 2nd edition.
Of course, October is also breast cancer awareness month, and Yale New Haven hospital issued with newsletter with advice on that critical topic.
Breast cancer is the second most common cancer among American women, except for skin cancer – but millions of women are surviving the disease, thanks in part to regular screening, early detection and improvements in treatment.
“Compared to 15 or 20 years ago, the proportion of early-stage breast cancers we are seeing in our clinics is significantly higher. We can directly attribute this to the improvements in screening technologies, in mammography, tomosynthesis, breast MRI, breast ultrasound and computer-assisted detection methods over the years,” said Meena Moran, MD, chief of Breast Radiation Oncology for the Smilow Cancer Network. “Another major factor attributing to earlier detection over the last two decades is the overall increased awareness of breast cancer and the importance of screening in the general population.”
From the miscellany department —
- The International Foundation of Employee Benefit Plans discusses “Optimizing Outcomes and Containing the Costs of Surgery.”
- Reg Jones writing in the Federal Times, provides the math on calculating Social Security benefits, especially early retirement benefits.