Midweek Update

OPM has released the 2020 highlights of its FEHB Plan Performance Assessment System. With this system, the 2020 plan scores which are based on 2019 data are used to determine the 2021 service charge for experience rated plans and the 2021 performance adjustment for community rated plans. 2020 was a tricky scoring year because the data was being gathered and analyzed just as the great hunkering down began in March 2020.
Reg Jones provide FEHB background for federal employees in Fedweek.
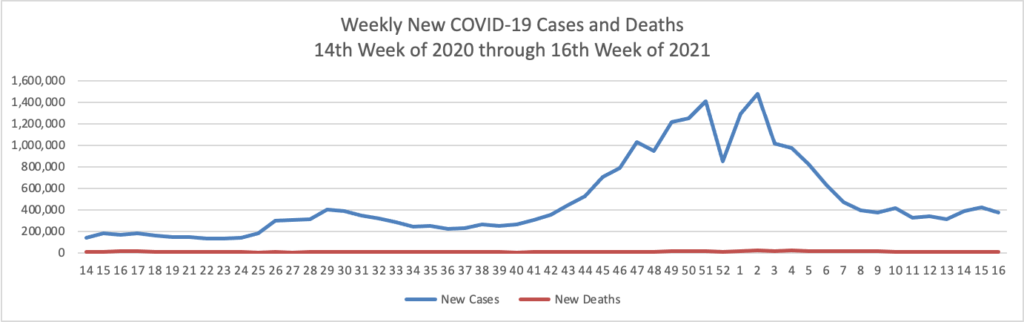
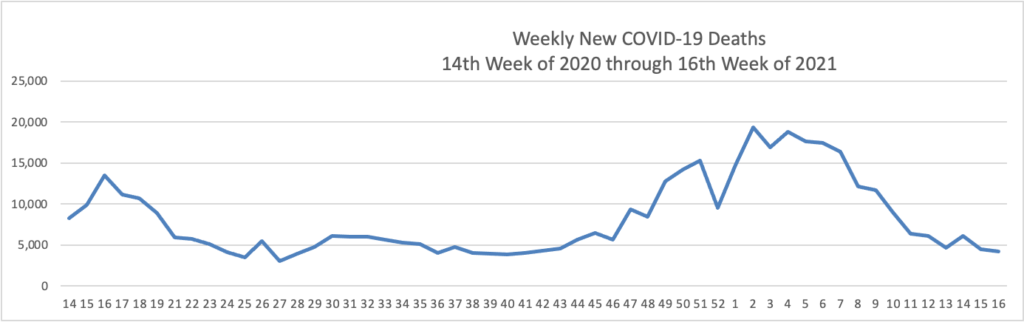
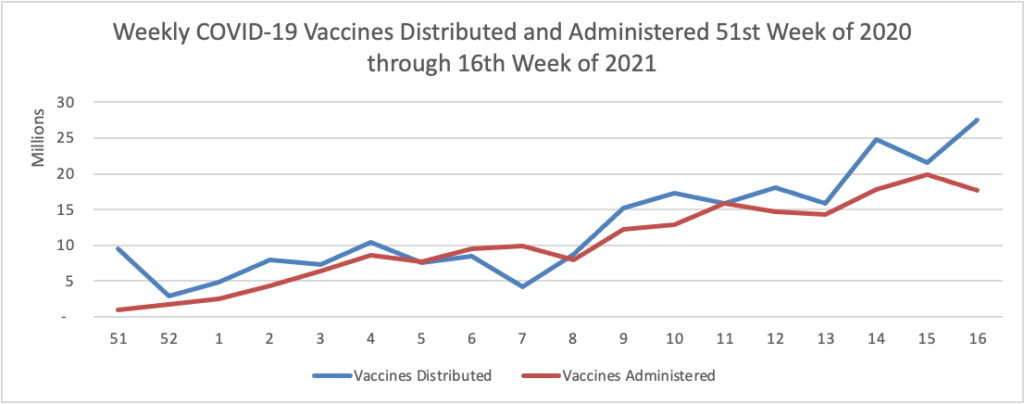
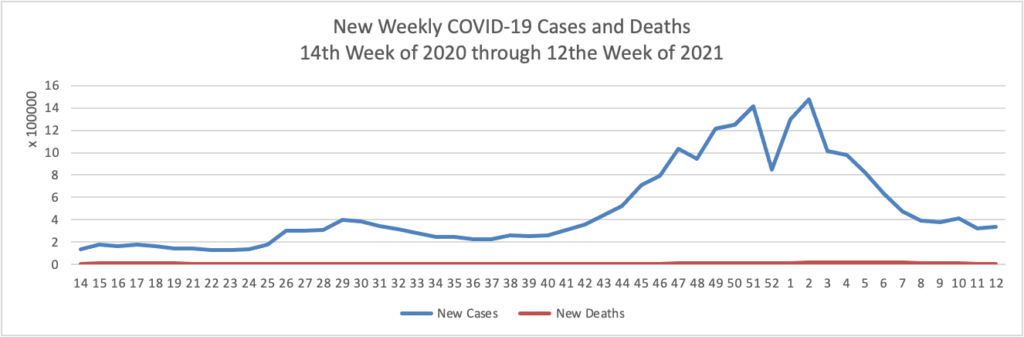
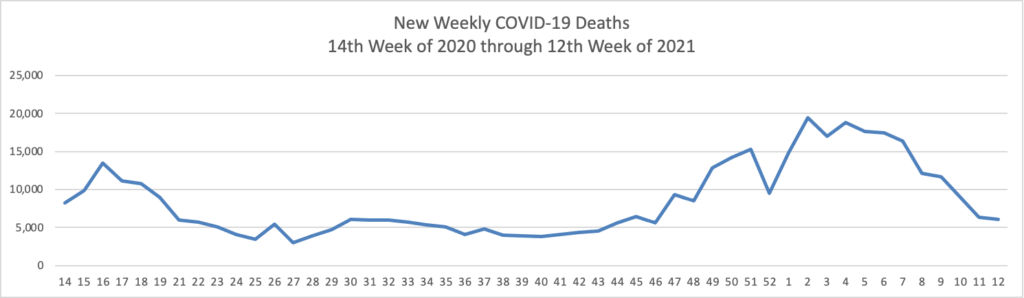
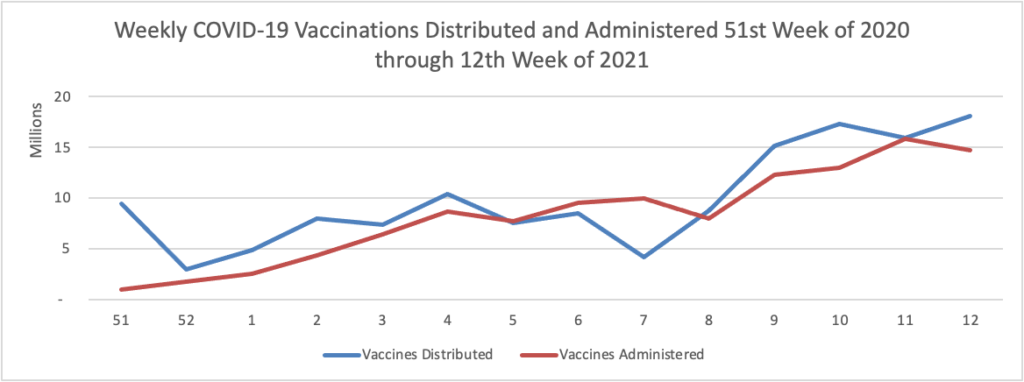
On the COVID-19 front, Bloomberg discusses the work of the federal government’s recently created COVID-19 Community Corps. The article discusses a Maine dairy farmer who set up a COVID-19 vaccination clinic for her employees and community members. “Organized in small teams that run the gamut from veterans and religious groups to progressive youth organizations and a Black LGBTQ group, the corps has been in the forefront of reaching the reluctant. The idea is that this wide demographic outreach will radiate, so that the friends and neighbors of the vaccinated follow suit.” Bravo.
WTOP, a local news radio station here in Washington DC reports that Pfizer “will seek [emergency] approval for use [of its COVID-19 vaccine] in children between 2 and 11 years old as early as September.
In other healthcare news
- Mobihealth News reports that “On-demand behavioral health platform Ginger is now available as a health benefit for Cigna’s 14 million members, the companies announced [on April 28]. Members with Cigna’s employer-sponsored or individual and family insurance plans can now access Ginger’s behavioral health coaching, therapy and psychiatry services as an in-network benefit.” Smart move.
- Healthcare Dive reports that “Telehealth utilization among the commercially insured fell 16% from January to February, the first month-to-month drop since September, according to a tracker from nonprofit Fair Health. The data suggests a potential slowdown in demand for virtual care services that spiked last year in the early months of COVID-19. Historically high levels of telehealth utilization spurred an unprecedented influx of cash into the digital health sector, but the sustainability of that boom depends in part on continued demand from consumers that could be waning as vaccinations increase and the pandemic wanes. Mental health conditions continued to top the list of diagnoses. However, COVID-19, which joined the top five diagnoses list in December, dropped from the list, likely reflecting the national decline in cases in February.”
- Since Monday the FEHBlog has been looking at the Health Affairs blog to post here Katie Keith’s follow up post on the 457 page long second final ACA notice of benefit and payment parameters. It turns out that he had posted Prof. Keith’s follow up post on Monday and that the lead entry was made last Saturday May 1. Here are the links to Prof. Keith’s lead and follow up posts on that important ACA rule making. Considering it’s Cinqo de Maio, lo siento lectors.
The FEHBlog hasn’t mentioned the Econtalk podcast in a while but he does listen every week. This week the host Russ Roberts spoke with behavioral scientist Katy Milkman of the Wharton School at the University of Pennsylvania talks about her new book How to Change: The Science of Getting from Where You Are to Where You Want to Be.” Professor Milkman talks about soft commitment strategies [to achieve goals] and hard commitment strategies. An example of a soft commitment strategy involved a doctor posting a letter visible to patients committing to specific Choosing Wisely recommendations such as proper prescribing of antibiotics. As for hard commitments she notes this example, which was news to the FEHBlog, “websites like StikK and Beeminder that let you fine yourself if you’re not achieving your goals.” She also discusses the carrot strategy .”The carrot is, let’s actually figure out ways to make it more enjoyable in the moment, and that way your willpower won’t be needed to do the thing that’s good for you.” An example is binging junk TV while using the treadmill. Their discussion on self control is fascinating. Check it out.