Friday Stats and More
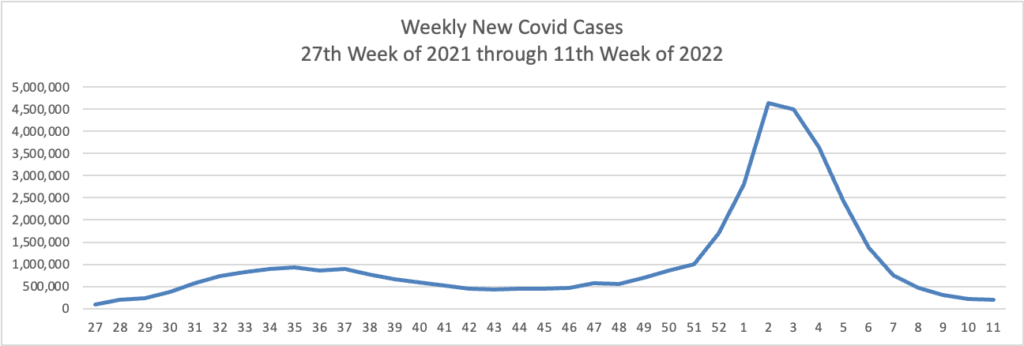
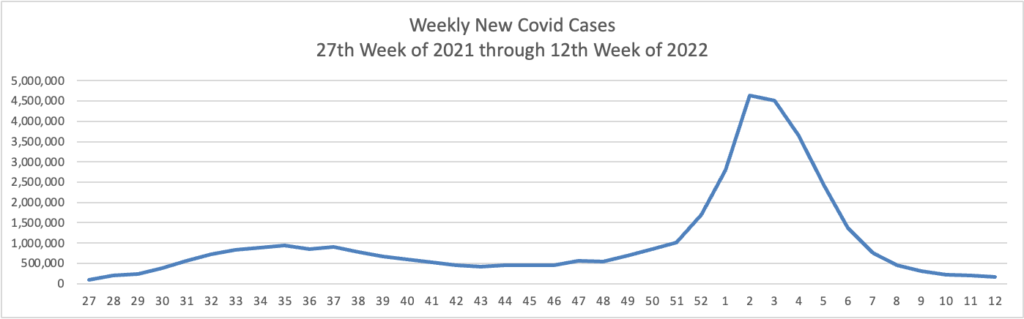
Based on the Centers for Disease Control’s Covid Data Tracker and using Thursday as the first day of the week, here is the FEHBlog’s weekly chart of new Covid cases from the 27th week of 2021 through the 12th week of 2022:
In the current CDC Covid Data Weekly Tracker, the CDC explains
In February, CDC’s COVID Data Tracker released a Wastewater Surveillance tab, which tracks changes and detections of SARS-CoV-2 viral RNA levels at more than 600 testing sites across the country. Because many people with COVID-19 shed the virus in their feces, wastewater testing can help us monitor COVID-19 in communities. Virus levels in wastewater usually increase four to six days before clinical cases increase, so surveillance results can help communities act quickly to prevent the spread of COVID-19.
Currently, virus levels in wastewater are relatively low across the country. More than half of all sites reporting wastewater data are experiencing a decrease in SARS-CoV-2 levels, but some have reported a modest uptick. These upticks may reflect minor increases from very low levels to levels that are still low. It’s important to note that even a small increase when levels are very low can appear like a dramatic increase in the percent change. However, there is a possibility that some communities might start to see an increase in COVID-19 cases. This could happen for a variety of reasons, like waning immunity, new circulating strains, and eased prevention strategies.
Right now, it’s too early to know if we’ll see a corresponding increase in reported cases across the country. Wastewater data are meant to be used with other COVID-19 surveillance data. CDC encourages local public health officials to watch for sustained increasing levels of the virus in wastewater, and to use wastewater surveillance data with other kinds of data to inform their decisions. CDC continues to encourage people to use COVID-19 Community Levels to find out what actions they should take to protect themselves and others. The whole community can be safe only when we all take steps to protect each other.
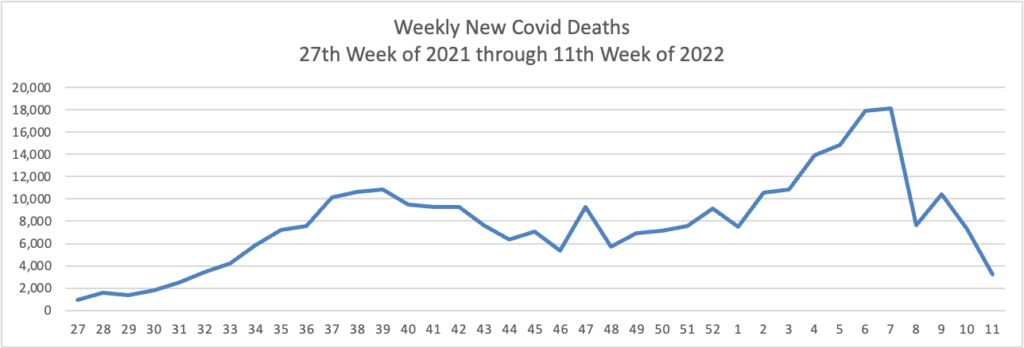
Using the same approach, here is the FEHBlog’s latest weekly chart of Covid deaths:
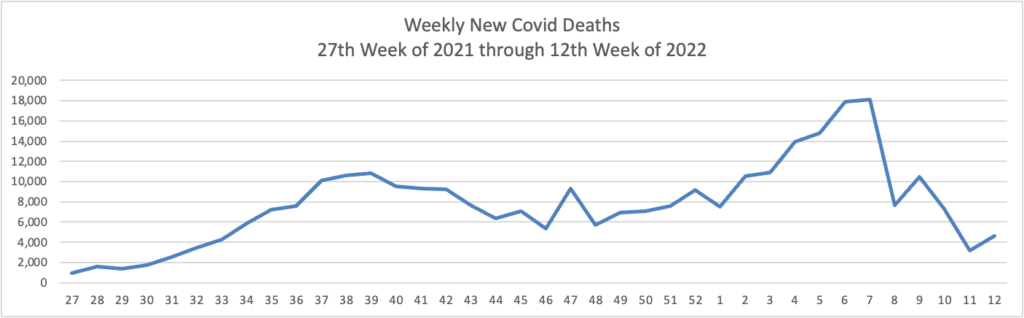
Precision Vaccinations adds that
[T]he 2022 trend data from the CDC indicates pneumonia may soon overtake COVID-19 as the leading cause of respiratory death in the U.S.
Historically, the CDC reported the number of visits to emergency departments with pneumonia as the primary diagnosis averaged about 1.5 million, which led to 47,000 deaths annually.
The good news is pneumonia is a vaccine-preventable disease.
Unfortunately, the percentage of adults who had ever received a pneumococcal vaccination was 25.5% in 2020.
Increasing the pneumococcal vaccination rate is a worthy goal for health plans and primary care providers.
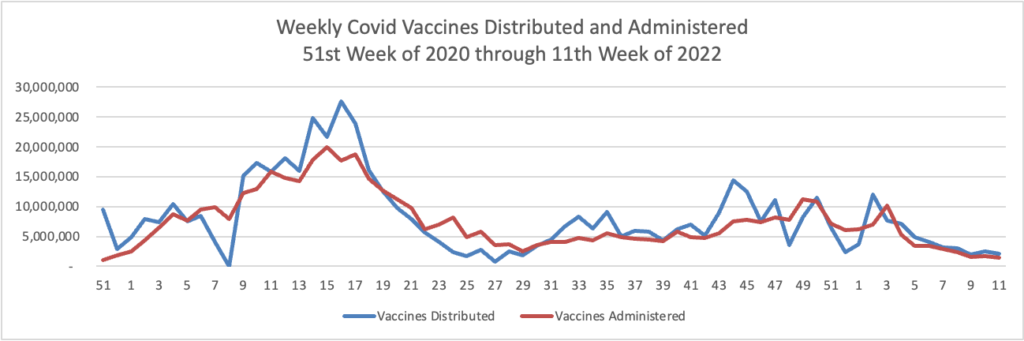
Here’s is the FEHBlog’s weekly chart of Covid vaccinations distributed and administered in the Covid vaccination era:
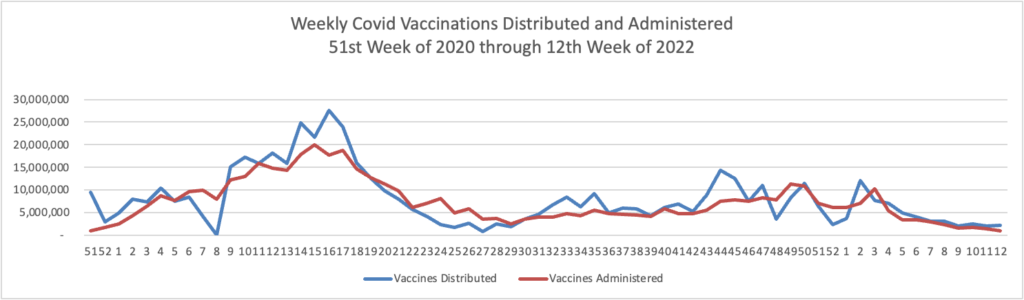
While recent vaccinations numbers are nothing to crow about, over 75% of Americans age 18 and older are fully vaccinated. Nearly half of the same population and over two-thirds of Americans age 65 and older have received a booster.
Politico adds
The Biden administration could authorize a second Covid-19 booster shot for older Americans within weeks, amid rising concern over a potential resurgence of cases, four people with knowledge of the matter told POLITICO.
The move under consideration by senior health officials would recommend the additional vaccine dose for adults 65 and older, in an effort to better protect high-risk people and stave off a wave of hospitalizations should infections climb rapidly as a result of the spread of the Omicron subvariant, BA.2. Currently, second boosters are only recommended for those with compromised immune systems.
From the No Surprises Act front, last Monday, Federal District Judge Richard Leon heard oral argument on dispositive cross-motions submitted by medical associations and the federal government regulators concerning the status of the qualifying payment amount in the baseball arbitration process. The FEHBlog has heard from a couple of sources who attended the hearing that Judge Leon indicated that he does not plan to put deciding the case on his front burner because the federal regulators advised him about their intent to issue the final, final rule on the Independent Dispute Review process in May 2022. The case is pending in the U.S. District Court for the District of Columbia.
From the telehealth front, mHealth Intelligence informs us
More than two-thirds of telehealth providers said they use audio-only modalities to offer telehealth services, according to a recent survey conducted by the American Medical Association.
The survey polled 2,232 physicians between Nov. 1 and Dec. 31, 2021.
The popularity of telehealth among physicians is apparent, with 85 percent saying they still use it. But 52 percent agreed that their telehealth usage has decreased since they first started offering the services. The top reason for the decrease was that they moved to a hybrid model of care with both in-person and virtual care services.
From the healthcare business front —
Fierce Healthcare reports
Optum has quietly acquired Refresh Mental Health from private equity firm Kelso & Company, Axios reported Thursday.
The company confirmed the deal in a statement to the outlet. The acquisition has not been announced publicly as of yet.
“Optum and Refresh Mental Health are excited to expand effective behavioral care to patients through a more coordinated health system,” the company said in a statement to Axios. * * *
Refresh was founded in 2017 and provides outpatient mental and behavioral health services. It runs 300 locations across 37 states that offer a variety of services including psychiatry and substance abuse treatment.
Bicycle Health, a virtual provider for opioid use disorder, is partnering with five additional payers, it said in an announcement provided exclusively to Fierce Healthcare.
The partnerships are with Molina Healthcare and McLaren Health Plans in Michigan, UHC Community Plan in Arizona, Health First Colorado (the state’s Medicaid program) and Blue Cross Blue Shield Texas. In total, these payer partnerships have the potential to reach more than 8 million patients, the company said. Coverage will include medication management, behavioral health (individual or group psychotherapy, medical care), support groups and care coordination.
“From high costs to significant time commitments, many traditional OUD recovery programs just aren’t realistic options for the vast majority of patient experiences,” Bicycle Health CEO and founder Ankit Gupta said in a statement. “We are committed to making science-based, holistic OUD treatment accessible to all who need it—and these partnerships are an exciting step towards that goal.”