Thursday Miscellany

From Capitol Hill, Roll Call reports
Lawmakers are facing increased pressure to pass a comprehensive mental health and substance use package but are unlikely to make an initial goal of advancing legislation before the implementation of a three-digit suicide hotline in July.
At least four congressional committees have committed to advancing a swath of bipartisan mental health bills under their jurisdiction, but lawmakers have not yet unlocked the puzzle of how to incorporate a growing laundry list of programs to authorize and establish existing and new programs dedicated to treatment, prevention, education, crisis care, drug interdiction and the workforce.
One of those four committees is the Senate Finance Committee which announced today
Senate Finance Committee Chair Ron Wyden (D-Ore.), Ranking Member Mike Crapo (R-Idaho), Senator Ben Cardin (D-Md.) and Senator John Thune (R-S.D.) today released a discussion draft for telehealth policies as a part of the committee’s ongoing work to improve mental health care across the nation, which has included a public call for comments and three hearings to help develop these initiatives. * * *
The discussion draft includes policies that would:
* Remove Medicare’s in-person visit requirement for tele-mental health services.
* Establish benefit transparency for mental health care services delivered via telehealth to inform Americans with Medicare how and when they can access telehealth.
* Preserve access to audio-only mental health coverage in Medicare when necessary and appropriate.
* Direct Medicare and Medicaid to promote and support provider use of telehealth.
* Incentivize states to use their CHIP programs to establish local solutions to serve behavioral health needs in schools, including through telehealth.
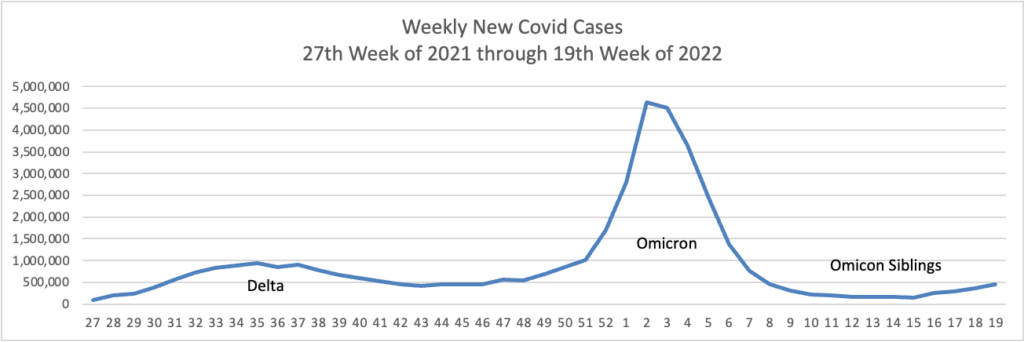
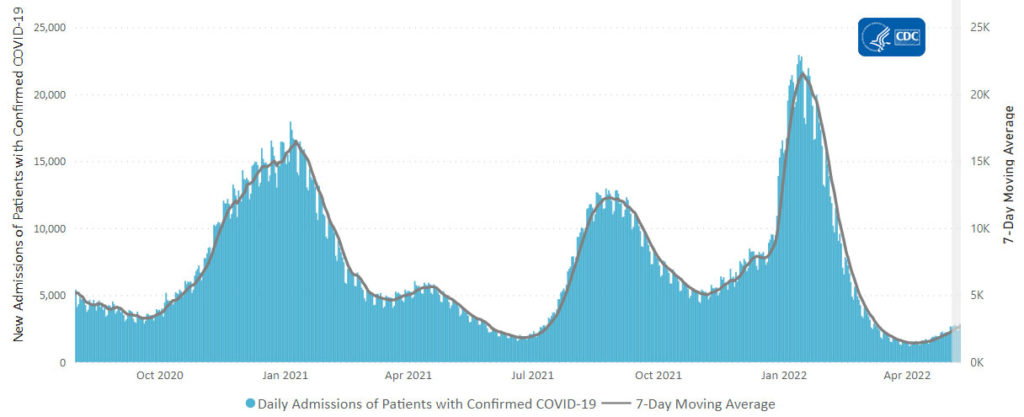
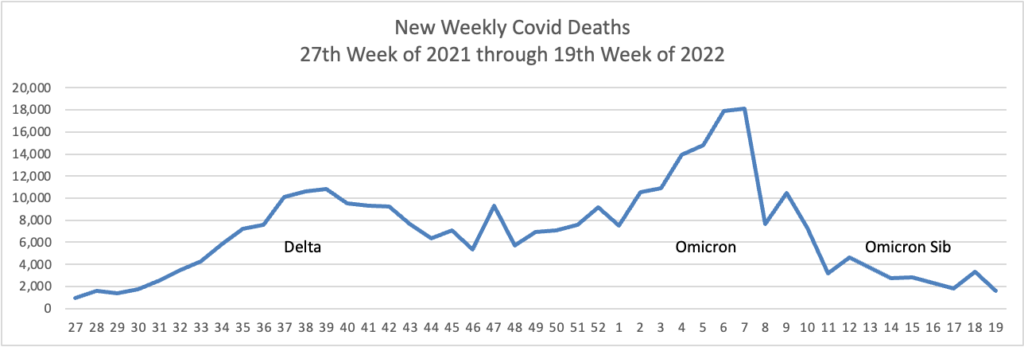
From the Omicron and siblings front
Beckers Hospital Review informs us
A highly contagious sublineage of the BA.2 omicron subvariant is now the nation’s dominant strain, according to the CDC’s latest variant proportion estimates.
The sublineage, BA.2.12.1, accounted for 57.9 percent of all U.S. COVID-19 cases in the week ending May 21, CDC data shows. BA.2, which became the nation’s dominant strain in mid-March, now accounts for an estimated 39.1 percent of all cases.
BA.2.12.1 is estimated to have a 25 percent growth advantage over BA.2, which is already more transmissible than the original omicron strain. The newer omicron sublineage has been gaining traction in the U.S. over the last month. In the week ending April 23, BA.2.12.1 accounted for just 24.1 percent of U.S. COVID-19 cases.
Health officials are also monitoring another omicron subvariant — BA.1.1.529 — which currently accounts for an estimated 2.8 percent of cases.
“Epidemiologically, it doesn’t appear as if we’re seeing more severe disease in places that are having more cases,” CDC Director Rochelle Walensky said of the sublineages during an April 26 news conference. “So we are not anticipating more severe disease from some of these subvariants, but we are actively studying it.”
CBS News reports
As many as one in four seniors and one in five adults under 65 experienced “long COVID” or “post-COVID” symptoms after surviving a coronavirus infection, a new study from the Centers for Disease Control and Prevention reported Tuesday.
The study — published in the CDC’s Morbidity and Mortality Weekly Report — is the latest to try and quantify how many of the millions of Americans who have now tested positive for the virus are facing long-term issues caused by their infection.
By comparing electronic health records in a large national database of patients, the study’s authors found 38.2% of COVID-19 survivors “experienced at least one incident condition” — a list that includes heart, lung, kidney and gastrointestinal problems, pain, fatigue, loss of smell or taste, mental health issues, and more — in the months after their infection. By contrast, just 16% of other people were diagnosed with such conditions.
The Wall Street Journal adds
Vaccination reduces your risk of developing long Covid, but not by much on average, new research suggests.
A Veterans Affairs study out Wednesday found that vaccinated people with breakthrough Covid-19 infections had a 15% reduction in experiencing persistent or new symptoms and health conditions up to six months after infection compared with those who were unvaccinated and got Covid.
Most of the vaccinated people had received two doses of the Pfizer or Moderna vaccine, while 8% received one dose of the Johnson & Johnson vaccine. The study didn’t look at people who had received boosters.
Bloomberg discusses the risks of contracting Covid while pregnant.
Canada’s first dual specialist in infectious diseases and obstetrics/gynecology, Deborah Money, MD comments “For the most part, women in communities even with Covid circulating do well,” she says. “The majority of babies are fine.”
But that’s just part of the story. Their analysis of data from 6,012 people in six Canadian provinces who tested positive for the virus during their pregnancy found a substantial increase in hospitalizations and ICU admissions compared with reproductive-age, non-pregnant females infected with the coronavirus. Their study in the May 2 issue of the JAMA medical journal also found that 11.1% of Covid–affected pregnancies resulted in preterm birth, compared with 6.8% among all unaffected Canadian pregnancies. * * *
Money says it underscores the need for obstetricians to carefully monitor their pregnant patients who become infected with SARS-CoV-2, and for expecting moms to get vaccinated and boosted.
“That’s the biggest thing they can do,” she says. “It really does look like vaccine is preventative for the serious outcomes.”
From the studies front —
- The Medical Group Management Association informs us “Despite multiple waves of disruption in 2021, medical practices navigated through the “new normal” of COVID-19 to restore a sense of normalcy in productivity and compensation last year.”
- Milliman released its 2022 Medical Index (MMI). “In 2022, the cost of healthcare for a hypothetical American family of four covered by an average employer sponsored PPO plan is $30,260,” 4.6% above 2021.
- HR Dive tells us “[a] 2019 IRS notice expanded the list of medications and health services Health Savings Account-eligible health plans may cover prior to meeting a patient’s deductible. Employers that take advantage of the expansion could cover these treatments with little to no increases in patient premiums, according to an Employee Benefits Research Institute report published May 19.”
- Fierce Healthcare reports
Commercial insurance members’ satisfaction with their plans stayed flat between 2021 and 2022, according to a new survey from J.D. Power.
Satisfaction was on a steady climb over the past five years, the survey found, but plateaued in the past year amid declines in how well members’ expectations for customer service were met and dissatisfaction with their plan designs and network providers.
Health plans that were perceived by members as responsive enjoyed higher scores than those that were not, J.D. Power found. The Kaiser Foundation Health Plan and regional Blues insurers were consistently ranked as the highest scoring in the study’s 22 geographic regions.
From the mental healthcare front, the International Foundation of Employee Benefit Plans notes
The U.S. Department of Labor (DOL) published new guidance on obtaining job protected leave under the Family and Medical Leave Act (FMLA) for workers seeking mental health support. The guidance clarifies that eligible employees are able to take FMLA leave for their own serious health condition or to care for a spouse, child or parent because of their serious health condition, and that a serious health condition can include a mental health condition.
The guidance includes:
* Fact Sheet #28O: Mental Health Conditions and the FMLA, and
* Frequently Asked Questions on the FMLA’s mental health provision
From the federal employee benefits front, benefits consultant Tammy Flanagan writes in Govexec about Federal Employee Group Life Insurance Program options for federal and postal annuitants. What’s more, Fedweek explains how FEHBP fills Medicare coverage gaps for those fine folks.