Thursday Miscellany

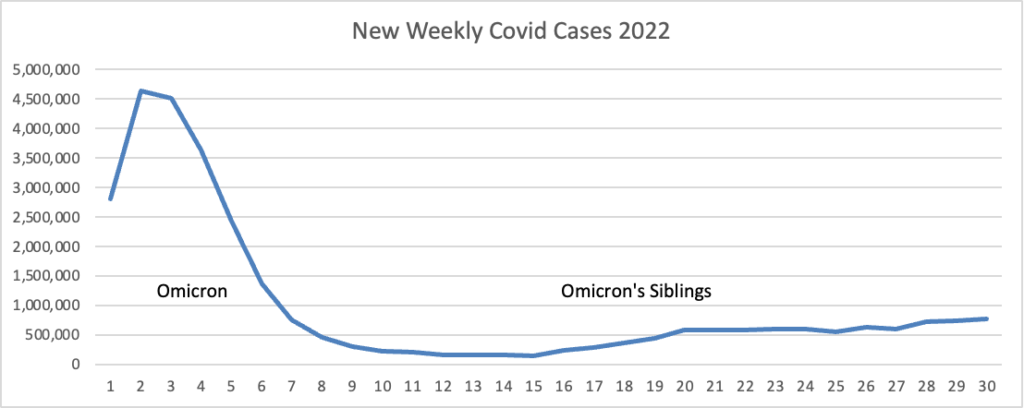
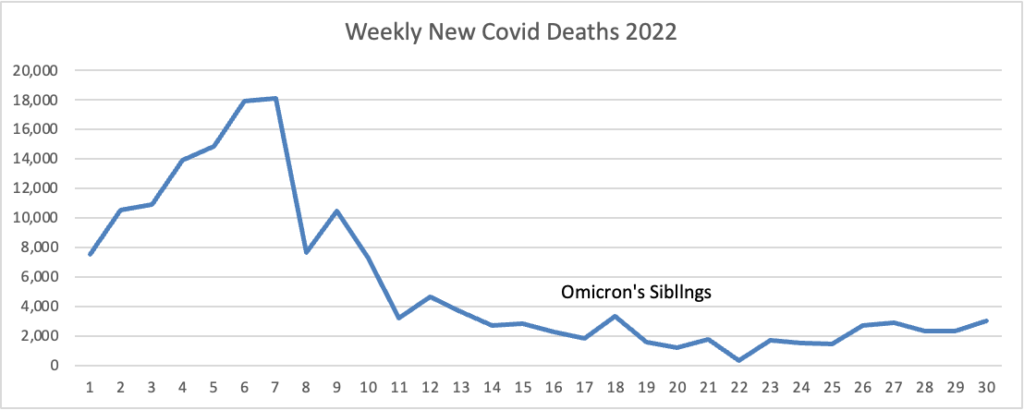
From the omicron and siblings front, AHIP informs us
Today the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted to recommend the use of the bivalent mRNA COVID-19 vaccines as a single-dose booster for all individuals ages 12 and older (Pfizer-BioNTech) and 18 and older (Moderna), at least two months following primary series or previous booster dose. The ACIP recommendation for the bivalent boosters was approved by a vote of 13-1.
The Committee reviewed modeling data showing the vaccine has the potential to reduce hospitalizations and deaths, especially among high-risk groups. The bivalent boosters are recommended for all those over age 12 who have completed a primary series, at least 2 months after the most recent dose. The modeling data indicates that the vaccine is safe and effective, and that booster strategies will be executed in an equitable manner. Models also indicate that waiting until more trial data is available (in two to three months) could lead to preventable hospitalizations and deaths.
ACIP members expressed concern that many assumptions had been made with the modeling, and that the vaccine being recommended – which includes protections specific to the BA.4/BA.5 variants – has not been tested in humans. Mouse models were used for data on this vaccine, in addition to extrapolations from human trials using the BA.1-specific vaccine. CDC pointed out that annual influenza vaccines are modified based on projected variants without direct clinical evidence. ACIP members also expressed concerns that the bivalent booster makes assumptions about future variants, which this booster may not protect against.
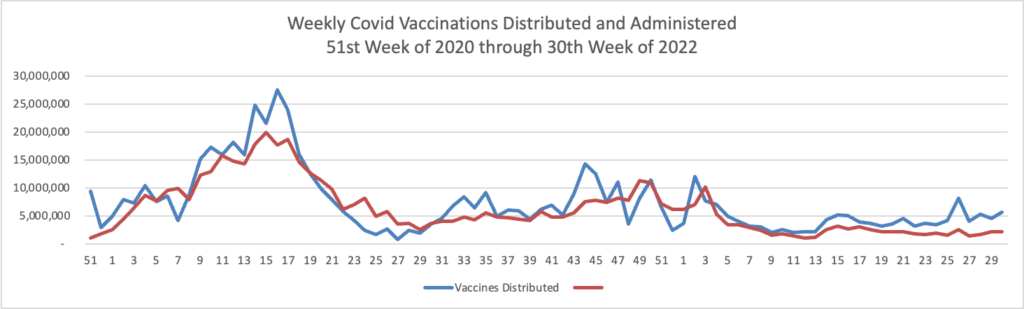
Earlier this week, the Food and Drug Administration (FDA) amended the Emergency Use Authorizations (EUAs) for both the Moderna COVID-19 vaccine and the Pfizer-BioNTech COVID-19 vaccine to authorize bivalent formulations of the vaccines for use as a single booster dose at least two months following primary or booster vaccination. The bivalent Moderna vaccine is authorized for use as a single booster dose in individuals 18 years of age and older and the Pfizer bivalent vaccine is authorized for use as a single booster dose in individuals 12 years of age and older. FDA also released fact sheets on both the Pfizer-BioNTech vaccine and the Moderna vaccine.
With the authorization, FDA has revised the EUAs to remove the use of the monovalent versions of the vaccines for booster administration for the age groups now covered by the bivalent booster products. ACIP also rescinded its recommendations for the monovalent booster vaccines.
Bivalent boosters may be available as early as next week.
The FEHBlog finds it noteworthy that the new bivalent booster replaces the monovalent booster.
STAT News offers FAQs on the new boosters.
STAT News also discusses the Omicron outlook for this autumn.
In a way, some physicians have said, Covid is becoming more like the other respiratory pathogens that most of us shake off but that can occasionally cause severe illness and death among the oldest adults or people who are already sick. So many more people are dying from Covid than from those other viruses, however, because of the massive number of cases that are still occurring overall.
Another trend that has continued into 2022 has been the racial and ethnic disparities associated with Covid. The gaps between different demographic groups’ death rates have shrunk over time, but at the peak of this summer’s wave, for example, death rates by age group among Hispanic adults were notably higher than those among white adults, federal data indicate.
One of the silver linings of the pandemic is that, unlike with some viruses, SARS-2 did not pose a particularly serious threat to children. That’s not to minimize the hospitalizations and deaths — as well as incidents of long Covid and MIS-C — that the virus did cause in pediatric populations. But overall, kids have faced much lower risks of severe outcomes from Covid than adults.
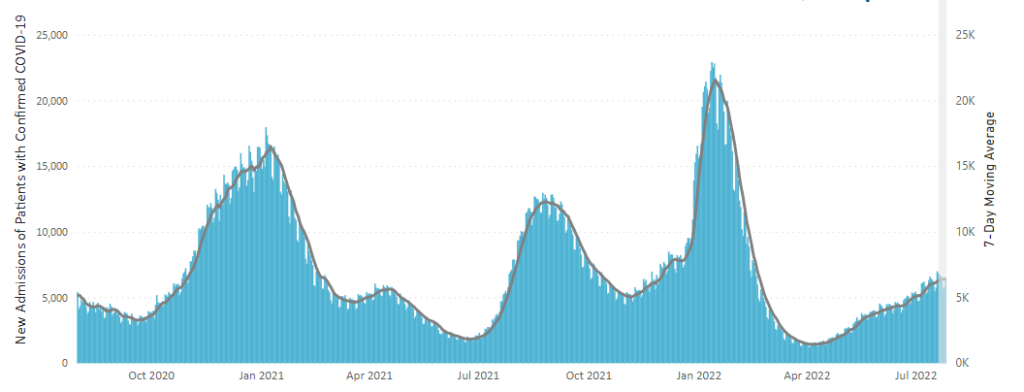
Still, something worrisome occurred this summer with kids and Covid, as hospitalizations reached their second highest peak of the entire pandemic, surpassing last summer’s Delta wave and only trailing the initial Omicron spike early this year.
The article’s experts encourage vaccinating children to stem this tide.
From the FEHB front, Govexec provides a handy just before and just after federal retirement checklist, and Fedweek helpfully delves into “What Counts and What Doesn’t for Keeping FEHB Coverage in Retirement,” which should be a key consideration for career feds.
From the public health front, the CDC reminds us September is Sepsis Awareness Month
Anyone can get an infection, and almost any infection, including COVID-19, can lead to sepsis. Sepsis is the body’s extreme response to an infection and is a life-threatening medical emergency.
September is Sepsis Awareness Month and CDC encourages patients and healthcare professionals to share Get Ahead of Sepsis resources, below, to learn how to protect themselves, their loved ones, and their patients from sepsis:
Patients and families:
* New this year is an updated patient and family brochure.
* Download and share any of CDC’s FREE patient education materials with your friends and loved ones to learn how to prevent infections, be alert to the signs and symptoms of sepsis, and act fast if sepsis is suspected.
* Share updated sepsis graphics on social media to educate friends and loved ones about sepsis.
* Are your children back to school? Talk to your child’s healthcare professional and school nurse about steps you can take to prevent infections that can lead to sepsis. Some steps include taking good care of chronic conditions and getting recommended vaccines.
Healthcare professionals:
New this year are two fact sheets for long-term care nurses and certified nurse assistants.
Download and share CDC’s FREE healthcare professional education materials with your colleagues to educate them about how to recognize signs and symptoms of worsening infection and sepsis, how to get ahead of sepsis, and what to do if they suspect sepsis.
Educate your patients and their families about:
o Preventing infections
o Keeping cuts clean and covered until healed
o Managing chronic conditions
o Recognizing early signs and symptoms of worsening infection and sepsis
o Seeking immediate care if signs and symptoms are presentThis Sepsis Awareness Month, spread the word about sepsis—you can help save lives.
To learn more about sepsis and how to prevent infections, visit www.cdc.gov/sepsis or call 1-800-CDC-INFO.
From the miscellany department —
- Medpage Today offers a special report about a ” New Behavioral Health Database Reveals Gaps in Care — Researchers behind it hope to provide the data needed to remedy the problem.”
- STAT News reports “Drug treatment of veterans with opioid use disorder increased during the first year of the pandemic, according to a new study, suggesting that the rapid shift from in-person to telehealth visits at VA medical centers enabled patients to get access to care despite Covid-related disruptions.”
- Fierce Healthcare tells us “Cigna’s Evernorth subsidiary is expanding its diabetes care value program to combine traditional pharmaceutical interventions with devices, tools and resources to help patients better understand and manage their diabetes.”
- Healthcare Dive informs us that “To staunch the losses of rural hospital closures that endanger access to care for millions, federal regulators are hoping some facilities opt in to a new payment model, but providers say they want more flexibilities and clarity before making the pivot. * * * The new rule ‘maybe gets halfway there,’ Jennifer Findley, vice president of education and special projects at the Kansas Hospital Association, told Healthcare Dive. ‘It’s not as much as we were hoping for but it does give some more flexibility than what you have today.’”
- Health Payer Intelligence reports “Chronic diseases are common among emergency department patients, particularly among seniors and those ages 45 to 64, according to a National Health Statistics Report. ‘Monitoring ED visits made by adults at highest risk of severe COVID-19-related illness is important for understanding the health burden of COVID-19 and for planning prevention strategies,’ the researchers explained. ‘Ongoing monitoring of the presence of these underlying chronic conditions at ED visits will continue to inform COVID-19 response efforts.’”
- Also Health Payer Intelligence notes that “Three major social determinants of health factors are particularly predominant barriers to care for America’s seniors: economic instability, loneliness, and food insecurity, according to a study sponsored by Alignment Healthcare. Researchers from Toluna conducted an online survey from July 24 to August 13, 2022, which reached 2,600 seniors ages 65 and older. Most respondents identified as white. Half were in Medicare Advantage and this population was divided primarily between preferred provider organizations and health maintenance organizations.”