Monday Roundup

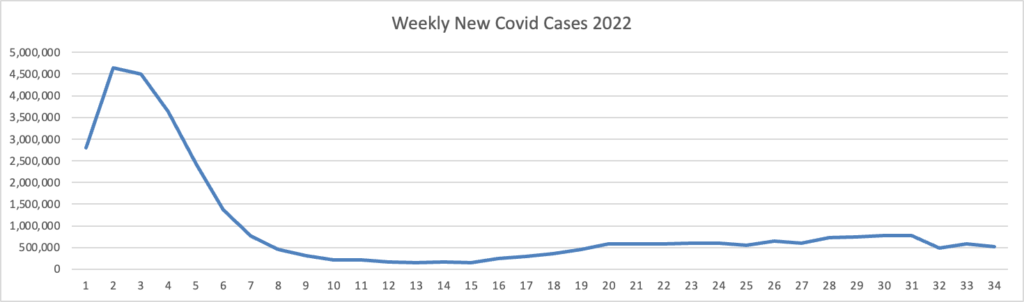
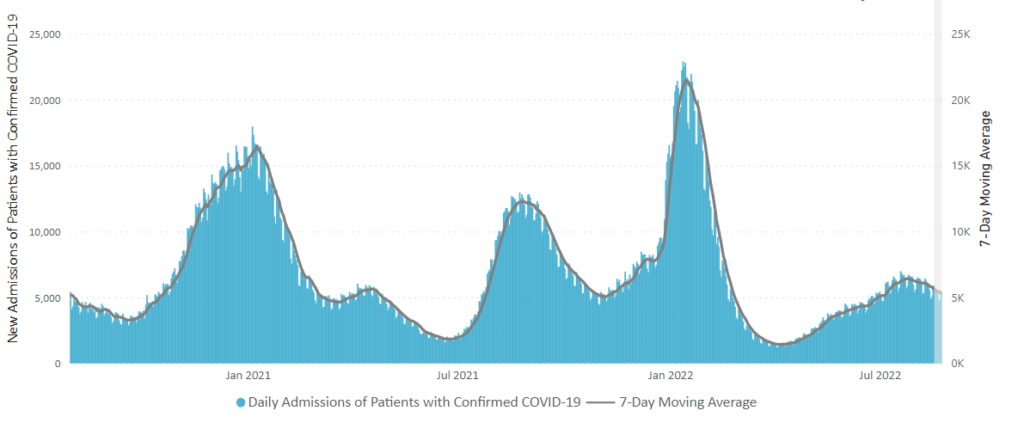
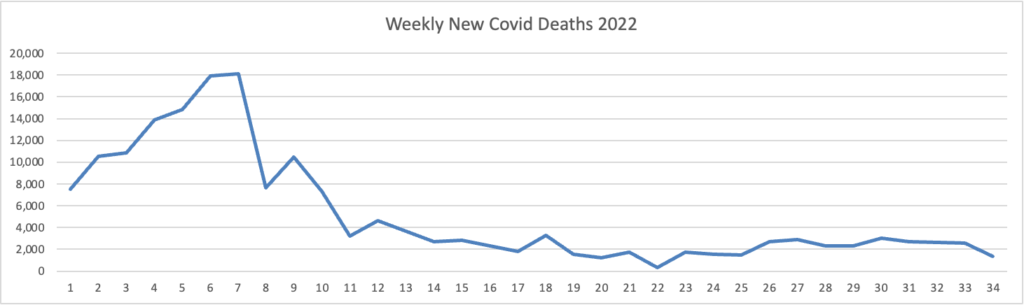
From the Omicron and siblings front, the Wall Street Journal reports
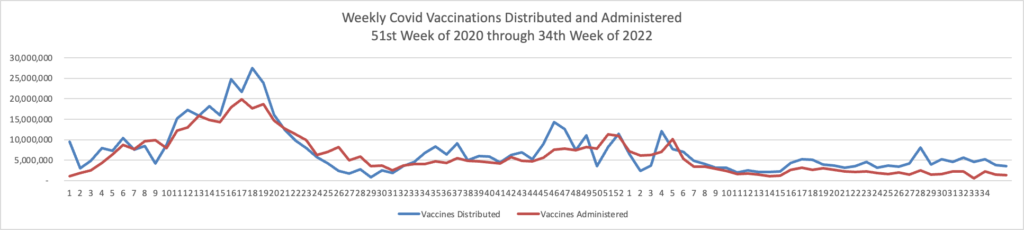
Most people would get one Covid-19 shot annually—as they do with the flu shot—under Food and Drug Administration proposals for simplifying the nation’s Covid-19 vaccine procedures.
The drug regulator also proposed that people getting vaccinated for the first time receive vaccines that target both Omicron and the original strain of the coronavirus.
The proposals, outlined in materials the FDA released Monday, would mark the biggest changes to Covid-19 vaccinations since boosters rolled out and are a sign of the nation’s shift to a more endemic-like approach to the coronavirus.
Vaccine experts who advise the FDA are scheduled to meet Thursday to discuss the proposals. The advisers are scheduled to vote on whether to give the bivalent shot as the initial inoculation, as is already allowed in Europe.
Makes sense to the FEHBlog.
From the OPM front, the House Oversight and Reform Committee Chairman James Comer (R-Ky.) has sent OPM Director Kiran Ahuja a letter demanding documents and a staff briefing on the recent GAO report criticizing OPM’s internal controls over family member eligibility in the FEHBP. Here’s a little free advice for my favorite agency. Rather than coming up with your own solutions, adopt solutions that have been proven to work in the private sector — the HIPAA 820 standard enrollment transaction which ties premium payments to enrollees and dependent eligibility verification audits based on statistical sampling.
From the U.S. healthcare business front —
Fierce Healthcare informs us
Elevance Health has inked a deal to acquire Blue Cross and Blue Shield of Louisiana, with the Pelican State insurer joining the Anthem Blue Cross affiliated plans.
The acquisition builds on an existing partnership between the two insurers, according to the announcement. The two jointly own Healthy Blue, a plan that serves Medicaid and dual-eligible beneficiaries.
The combination will also allow BCBSLA to accelerate its push toward improved access, affordability and quality for its 1.9 million members, thanks to the capabilities of Elevance Health’s Carelon subsidiary, the companies said. More than $4 billion has been invested in Carelon over the past several years, building out its behavioral health, complex and chronic care programs and digital health models.
and
CVS Health has named two key leaders for its pharmacy and consumer products business, including a returning face to the company, according to a report from Bloomberg.
David Joyner, a former executive at the company, will make a return as the leader of its pharmacy services segment, which includes the Caremark pharmacy benefit manager, people familiar with the matter told the outlet. Joyner left CVS three years ago and will succeed Alan Lotvin, M.D., who is set to retire.
In addition, former Express Scripts President Amy Bricker will join the company as the chief product officer for the consumer segment, which centers on developing new products for CVS’ consumer health brands, Bloomberg reported.
Fierce Healthcare points out a twist in the second story.
That Bricker had departed Express Scripts, a subsidiary of Cigna, was revealed last week when the PBM announced it had named a new president, veteran supply chain leader Adam Kautzner. What was next for Bricker, however, was conspicuously absent from the announcement.
The FEHBlog often counsels clients on Family and Medical Leave Act issues. He had no idea until today that the Labor Department offers helpful information to healthcare provider and employees on this law. For example,
This background information can fill knowledge gaps for employers too.
From the Rx coverage front —
- The Washington Post reports on the reaction to “the American Academy of Pediatrics guidelines, based on decades of scientific research, call[ing] for early and aggressive treatment, instead of “watchful waiting.” They urge intensive therapy for children as young as 6, weight loss drugs for those as young as 12 and surgery for teens as young as 13.”
- The Institute for Clinical and Economic Research released a
Final Evidence Report on Fezolinetant for Vasomotor Symptoms Associated with Menopause
— Independent appraisal committee voted that evidence is not yet adequate to demonstrate a net health benefit for fezolinetant when compared to no pharmacological treatment —
— Using point estimates from short-term clinical trials, analyses suggest this drug would achieve common thresholds for cost-effectiveness if priced between $2,000 – $2,600 per year for women who cannot or choose not to take menopausal hormone therapy —
— All stakeholders have a responsibility and an important role to play in ensuring that women have access to effective new treatment options for symptoms of menopause
The ICER upshot is “Given that many patients may benefit from readily available, effective, and low cost [menopausal hormone therapy] MHT, clinical experts agreed that it would be reasonable for payers to require prescriber attestation that patients are not appropriate candidates for MHT prior to prescribing fezolinetant.”
From the SDOH front, Health Leaders Media tells us about new ICD-10 diagnosis codes with an SDOH emphasis which will take effect on April 1, 2023.
From the telehealth front, U.S. News reports,
Despite distance and occasional technical glitches, a new study finds that most patients like seeing a surgeon for the first time via video.
The study was published Jan. 19 in the Journal of the American College of Surgeons. * * *
The study included 387 patients who participated in first-time visits between May 2021 and June 2022 at general surgery clinics across the Vanderbilt system. Researchers used a standard questionnaire to look at the quality of shared decision-making and asked patients and surgeons open-ended questions about their consultations.
In all, 77.8% of patients had an in-person visit, while 22.2% saw their doctor remotely.
Both groups reported high levels of quality communication during these appointments.
Levels of shared decision-making and quality of communication were similar between remote visits and in-person care, the study found.
In responding to the open-ended questions, patients praised the convenience and usefulness of telehealth appointments. Researchers received some negative comments about technical difficulties and not being physically present.