Friday Stats and More
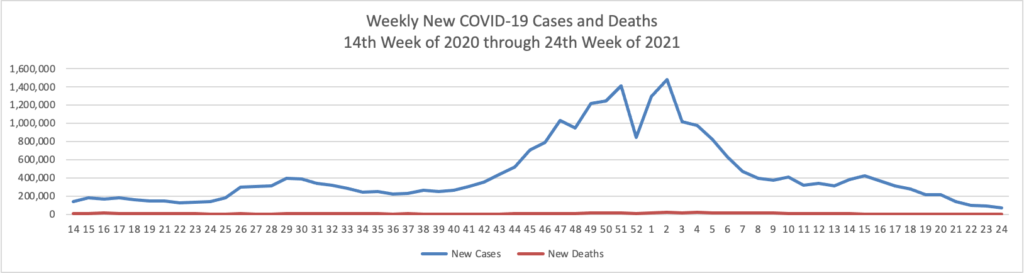
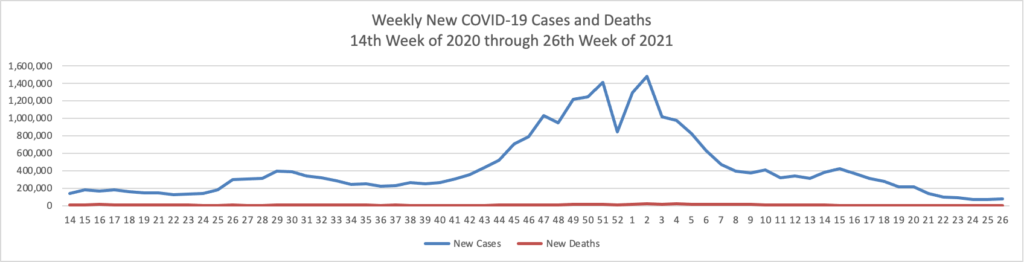
Based on the Centers for Disease Control’s (“CDC”) improved COVID-19 Data Tracker website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through 26th week of this year (beginning April 2, 2020, and ending June 30, 2021; using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

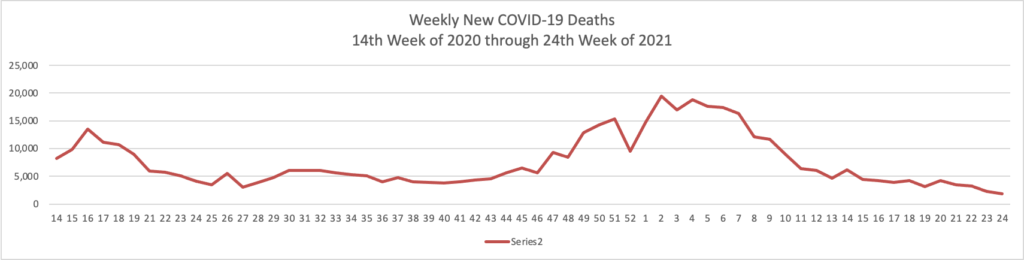
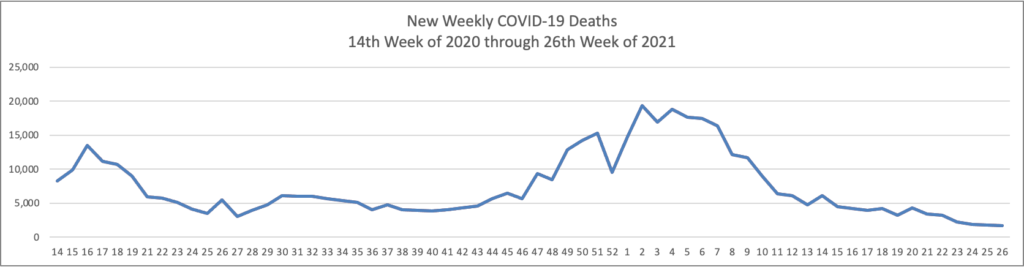
The FEHBlog has noticed that the new cases and deaths chart shows a flat line for new weekly deaths because new cases significantly exceed new deaths. Accordingly here is a chart of new COVID-19 deaths over the period (April 2, 2020, through June 30, 2022):
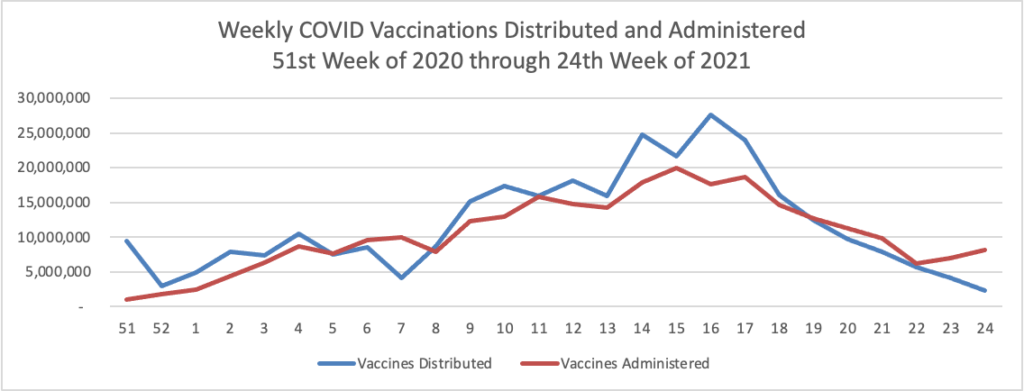
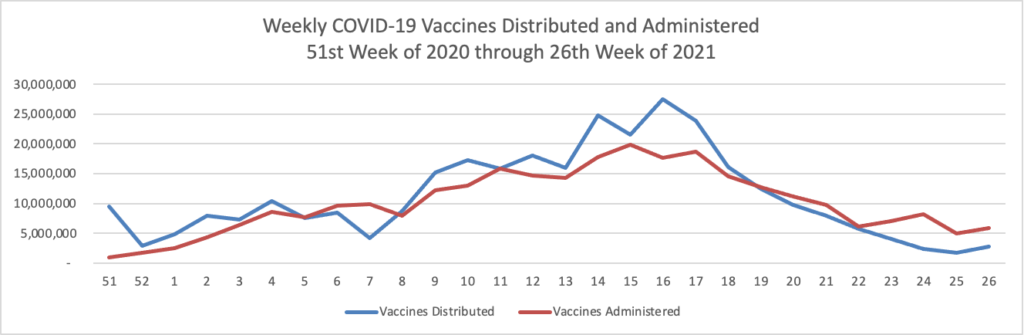
Finally here is a COVID-19 vaccinations chart over the period December 17, 2020, through June 30, 2022 which also uses Thursday as the first day of the week:
In its weekly COVID-19 update the CDC reminds us that
The emergence and spread of variants also have the potential to chip away at our nation’s progress to end this pandemic. On June 15, 2021, CDC announced classification of the SARS-CoV-2 variant B.1.617.2 (Delta) as a variant of concern because it spreads more easily. The spread of the more transmissible B.1.617.2 variant combined with the U.S. population that remains unvaccinated leaves many people at risk of infection. With B.1.617.2 now spreading across the country and infecting people worldwide, it’s more important than ever that all eligible people get vaccinated as soon as possible.
The COVID-19 vaccines authorized for use in the United States offer protection against all known variants, including the B.1.617.2 variant. Getting vaccinated will protect you and the people you love. COVID-19 vaccines are free and available for everyone ages 12 and up. They are nearly 100% effective against severe disease and death, meaning nearly every death due to COVID-19 is preventable. No matter your age, or your health history, until you’re fully vaccinated*—you are at risk of infection. By getting vaccinated and encouraging those around you to do the same, you can safely engage in activities you enjoyed prior to the COVID-19 pandemic. Get vaccinated, help others get vaccinated, and use prevention measures if not fully vaccinated so we can all celebrate our freedom from the virus.
Also from the COVID-19 front Becker’s Payer Issues reports that
The Vaccine Community Connectors program, which was launched by AHIP and the Blue Cross Blue Shield Association, has helped vaccinate more than 2 million people over 65 against COVID-19 in under 100 days, according to a July 1 news release.
More than 50 health insurers are now participating in the program, which was initiated to expand vaccination efforts of people in low-income communities.
“The most vulnerable people in our country have suffered disproportionately from the COVID-19 crisis, which is why we have been working side-by-side with industry partners to help millions of vulnerable Americans get vaccinated against the virus,” BCBSA CEO Kim Keck said in the statement.
Bravo! Health Payer Intelligence reports on how employers can best assist the nationwide vaccination campaign.
Seventy-three percent of workers whose employers encouraged them to receive the coronavirus vaccine had at least one vaccine shot. Additionally, 75 percent of those who received paid time off to get the vaccine have gotten at least one shot.
In contrast, only four in ten of those whose employers did not encourage employees to get vaccinated had received a coronavirus vaccine shot. Similarly, half of those whose employers did not provide paid time off to get the vaccine reported that they had received the coronavirus vaccine.
These distinctions remained true regardless of variations in race, age, ethnicity, income, and even regardless of whether the individual identified as Republican or Democrat.
In the more news category:
- The U.S. Supreme Court today added two healthcare cases to its October 2021 docket. According to Law360, the Court “agreed to review whether the federal government lawfully cut billions of dollars from reimbursements for drugs bought through a discount program for hospitals in low-income areas, as well as a reimbursement calculation for hospitals that serve a high amount of low-income individuals.”
- Benefits Pro reports that “Under pressure to rein in skyrocketing prescription drug costs, states are targeting companies that serve as conduits for drug manufacturers, health insurers and pharmacies. More than 100 separate bills regulating those companies, known as pharmacy benefit managers, have been introduced in 42 states this year, according to the National Academy for State Health Policy, which crafts model legislation on the topic. The flood of bills comes after a U.S. Supreme Court ruling late last year backed Arkansas’ right to enforce rules on the companies. At least 12 of the states have adopted new oversight laws. But it’s not yet clear how much money consumers will save immediately, if at all.” The source of the cost problem is not the PBMs who work with health plans to control costs. The PBMs vastly expanded prescription drug coverage for Americans. The sources of the cost problem are the manufacturers and wholesales. All these new state law simply adds more costs to the drug distribution process in the FEHBlog’s view.
- Becker’s Payer Issues explains how the BCBSA chief innovation officer seeks to transform healthcare one step at a time. Good luck to her. The FEHBlog loves her title.