Monday Roundup

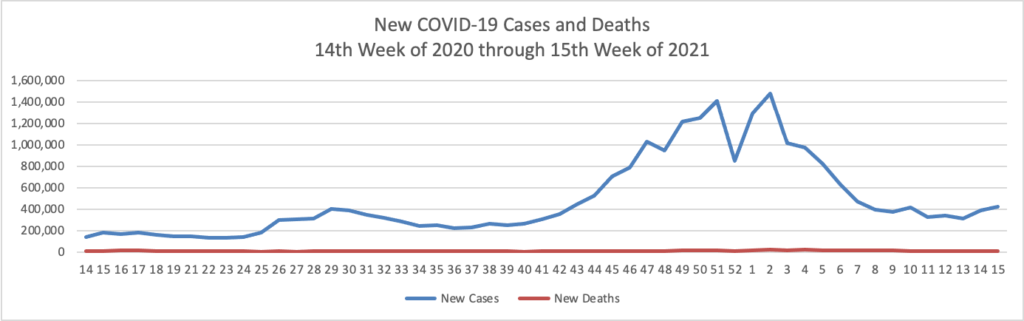
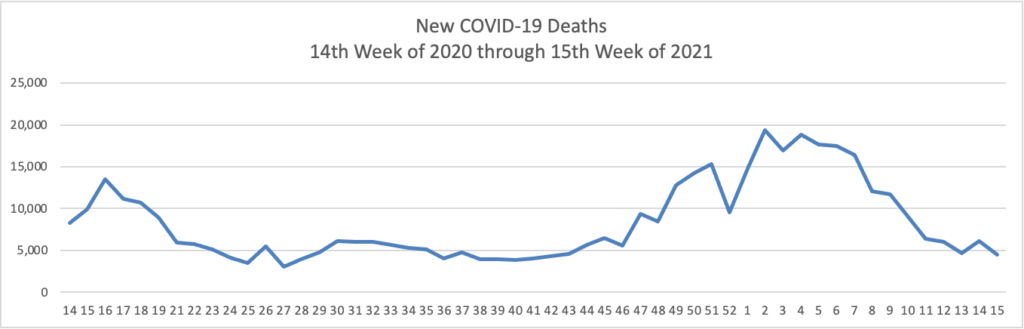
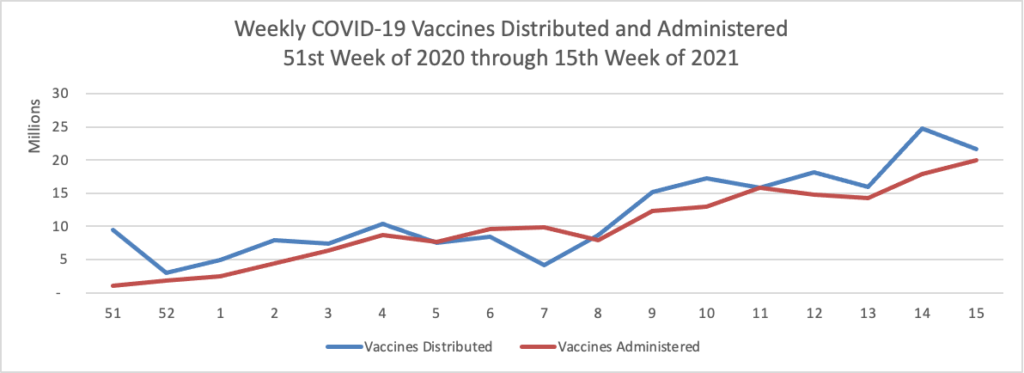
Mondays have tended to be good news days for COVID-19 vaccines. As of today, over 50% of Americans over age 18 have received at least one dose of a COVID-19 vaccine.
Fierce Healthcare reports that
“CVS Pharmacy has begun stocking its virtual and in-store shelves nationwide with rapid tests for COVID-19—which can be purchased without a prescription and used by anyone regardless of whether or not they are showing symptoms—including three FDA-authorized diagnostics and sample collection kits produced by LabCorp, Ellume and Abbott.”
“Even as vaccines become more widely available, COVID-19 testing remains a critical tool to keep our communities safe,” Walgreens President John Standley said in a statement. Walgreens currently offers on-site testing at more than 5,500 of its pharmacies and plans to expand to 6,000 drive-thru sites by May, using Abbott’s ID NOW portable testing machines.
In addition, earlier this month CVS began offering COVID-19 antibody testing for $38 at 1,100 in-house clinics, using fingerstick blood samples to determine previous infections.
The U.S. Office of Personnel Management announced today that the agency
will allow [FSAFEDS] flexibilities permitted under the Consolidated Appropriations Act 2021 and the American Rescue Plan Act including allowing full carryover for a health care flexible spending account (HCFSA) and Limited Expense FSA (LEX FSA); extending the grace period for a dependent care flexible spending account (DCFSA); and permitting care for dependents through age 14 for 2020 and 2021 under a DCFSA. In addition, OPM is working with our FSAFEDS contractor, Health Equity, to offer a Special Enrollment/Election Period (SEP) in the near future. This SEP will allow participants to increase or decrease their current elections for their DCFSA and/or their HCFSA. In addition, the SEP will allow those who did not re-enroll for 2021 during Open Season in the Fall, the opportunity to enroll in a DCFSA and/or HCFSA for 2021. Finally, OPM will allow DCFSA participants to increase their election during the Special Election Period to the new IRS maximum of $10,500 for 2021.
All good news.
What’s more, the Wall Street Journal reported in its Saturday essay about the U.S. airline safety revolution.
Over the past 12 years, U.S. airlines have accomplished an astonishing feat: carrying more than eight billion passengers without a fatal crash.
Such numbers were once unimaginable, even among the most optimistic safety experts. But now, pilots for domestic carriers can expect to go through an entire career without experiencing a single engine malfunction or failure. Official statistics show that in recent years, the riskiest part of any airline trip in the U.S. is when aircraft wheels are on the ground, on runways or taxiways.
The achievements stem from a sweeping safety reassessment—a virtual revolution in thinking—sparked by a small band of senior federal regulators, top industry executives and pilots-union leaders after a series of high-profile fatal crashes in the mid-1990s. To combat common industry hazards, they teamed up to launch voluntary incident reporting programs with carriers sharing data and no punishment for airlines or aviators when mistakes were uncovered.
One wonders whether this successful strategy may be transferable to other pressing safety issues, such as patient safety. In this regard, a friend of the FEHBlog suggested check this Washington Post opinion piece written by a group of psychologists titled “We instinctively add on new features and fixes. Why don’t we subtract instead?
‘Less is more’ is a hard insight to act on, it turns out.” How true.
In other healthcare news —
- The Kaiser Family Foundation informs us that
a relatively small number and share of drugs accounted for a disproportionate share of Medicare Part B and Part D prescription drug spending in 2019 (Figure 1).
— The 250 top-selling drugs in Medicare Part D with one manufacturer and no generic or biosimilar competition (7% of all Part D covered drugs) accounted for 60% of net total Part D spending.
— The top 50 drugs covered under Medicare Part B (8.5% of all Part B covered drugs) accounted for 80% of total Part B drug spending.
Some recent proposals to lower prescription drug prices have limited the number of drugs subject to price negotiation and international reference pricing. This analysis shows that Medicare Part D and Part B spending is highly concentrated among a relatively small share of covered drugs, mainly those without generic or biosimilar competitors. Focusing drug price negotiation or reference pricing on a subset of drugs that account for a disproportionate share of spending would be an efficient use of administrative resources . . . .
- Employee Benefits News tells us
New research from Voya shows employees have a bias against HDHPs and the reason for that is as simple as marketing.
“One of the really interesting findings that we saw from the research about why there is that bias comes down to branding, pure and simple,” says Nate Black, vice president of consumer driven health for Voya Financial. “When we replaced the high deductible health plan name and called it something more generic, the share of people choosing high deductible health plans doubled. So just the name itself can have a really significant impact on how people think about what plan they should choose.”
Sixty-three percent of the people surveyed by Voya said they would choose the plan with the lowest deductible. As part of the study Voya designed an experiment asking participants to choose between a PPO and an HDHP. The experiment was set up in a way that the HDHP was always the optimal financial choice, despite this, 65% of those surveyed still chose the PPO plan.
Communicating the long term value of plans connected with health savings accounts is quite important.
- Here’s a link to the CDC’s website on the Johnson & Johnson vaccine pause which explains
If you received the vaccine more than three weeks ago, the risk of developing a blood clot is likely very low at this time.
If you received the vaccine within the last three weeks, your risk of developing a blood clot is also very low and that risk will decrease over time.
Contact your healthcare provider and seek medical treatment urgently if you develop any of the following symptoms: severe headache, backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin (petechiae), or new or easy bruising.
If you experience any adverse events after vaccination, report them to v-safe and the Vaccine Adverse Event Reporting System
The FEHBlog enrolled in v-safe after his first Pfizer vaccination and the CDC has continued to inobtrusively check in weekly. The FEHBlog is happy to help out.