Memorial Day Weekend update

Congress is on a State / district work break this coming week. The Supreme Court has over thirty cases to decide, including the latest Affordable Care Act constitutionality case, before adjourning for the summer in late June / early July.
The federal employee news organizations have highlighted portions of the President’s fiscal year 2022 budget proposal which was released last Friday.
- The Federal Times reports that “President Joe Biden’s fiscal year 2022 budget anticipates a more than 50,000 full-time-equivalent employee increase to the federal payrolls next year, as part of concerted efforts to attract young and expert workers to federal service. * * * ‘The Federal workforce continues to become older on average. Almost 30 percent (635,397) of employees are older than 55, while 8.1 percent (176,805) of employees are younger than 30. By comparison, in the private sector, 23 percent of the workforce is younger than 30. Every single agency has fewer employees younger than 30 today than they had in 2010,’ the budget proposal’s analytical perspectives state.
- Govexec informs us that “President Biden on Friday formally proposed an average 2.7% pay increase for federal civilian employees in 2022 as part of his fiscal 2022 budget proposal. * * * It was unclear Friday how Biden’s proposal would be divvied up between an across-the-board boost to basic pay and increases in locality pay. In recent years, pay raise provisions have included a 0.5% average increase in locality pay, although it was frozen at 2020 levels this year. * * * The proposal also marks a return to the principle of pay parity between the civilian and military workforce, as service members would also receive a 2.7% pay raise in 2022.
Federal News Network offers three brief stories on the U.S. Postal Service
The Postal Service sent its first reduction in force notices to non-union management employees Friday [no indication of how many notices were sent out], and is planning to set higher prices on its mail products well above the rate of inflation [first class stamp would increase by 5% from 55 cents to 58 cents].
The Senate, meanwhile, voted [by unanimous consent] to confirm [Anton Hajjar] President Joe Biden’s third nominee to serve on the USPS Board of Governors. The board is now fully staffed for the first time since 2010, and will help the agency get its 10-year reform plan off the ground.
Thus the Senate has confirmed all three of the President’s Postal Governor nominees who shared a confirmation hearing with OPM Director Kiran Ahuja while Ms. Ahuja waits for a confirmation vote.
In healthcare news —
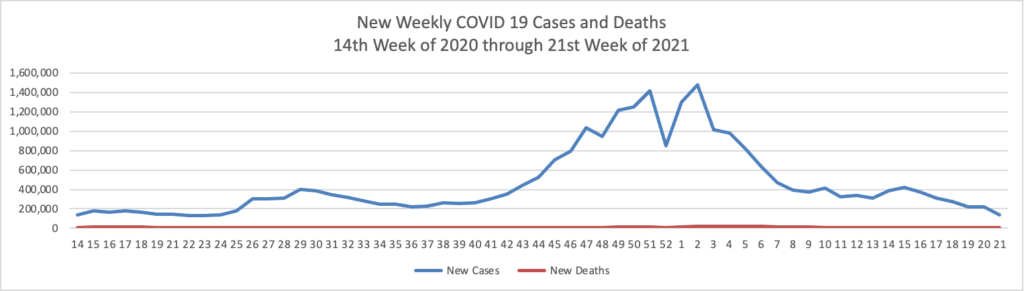
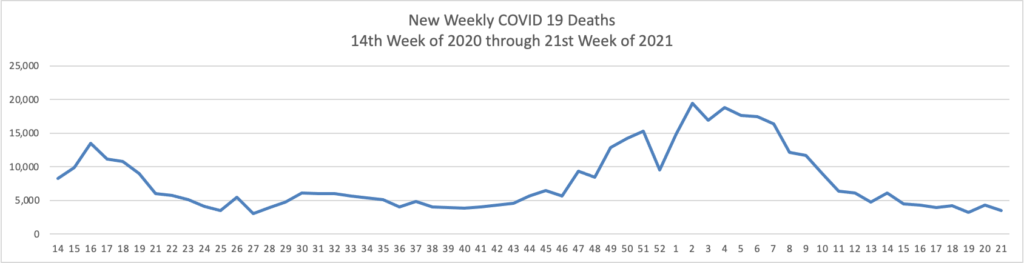
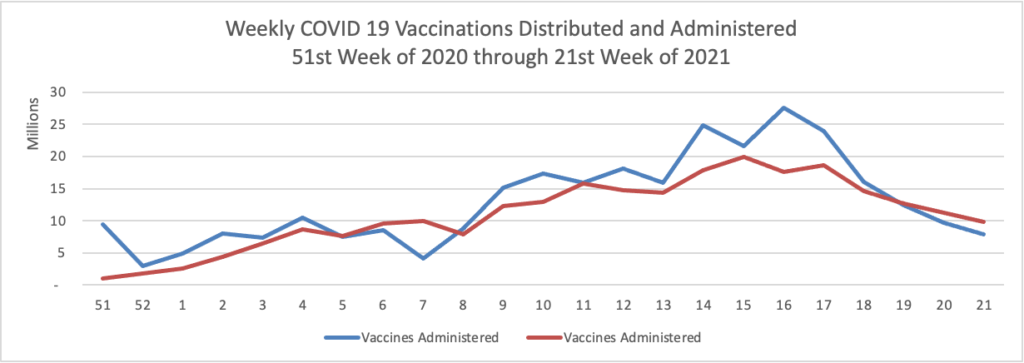
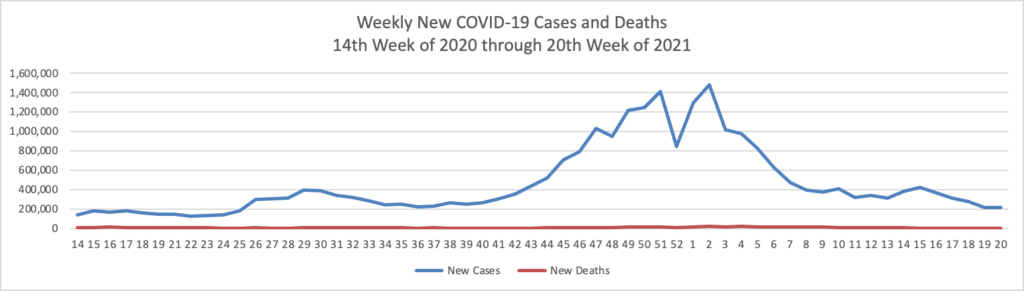
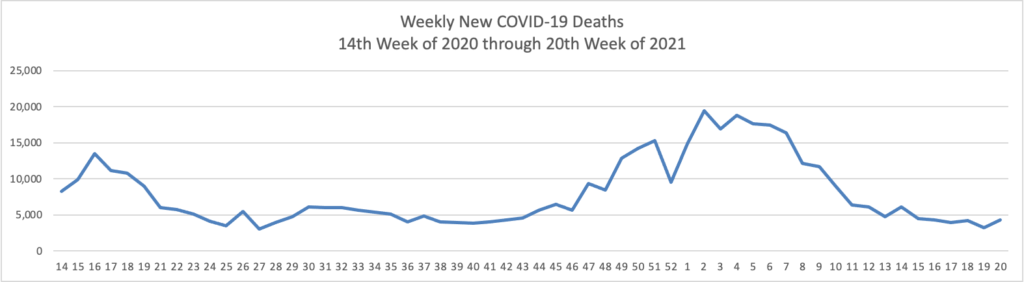
- Bloomberg tells us that “The U.S. reported the lowest level of infection since the early days of the pandemic and welcomed back sports fans to stadiums. The Indianapolis 500 was run before 135,000 fans, the largest crowd for a sporting event since the pandemic began [but 1/3 of capacity]. * * * The world needs the cooperation of the Chinese government to trace the origins of Covid-19 and prevent future pandemic threats, two leading U.S. disease experts said Sunday.
- NPR reports that “For children in particular, the risk of serious consequences from COVID-19 is the same magnitude as the risk they face from the flu, she says. But many parents seem more worried about the new and less familiar disease. * * * [E]xperts urge parents to try not to worry too much.
- Because many FEHB plans provide hypertensive members with at home blood pressure monitor, the FEHBlog wants to share the American Medical Association’s views on what doctors wish their patients knew about home blood pressure measurement.
Finally, the FEHBlog’s eyes were drawn to the weekend Wall Street Journal’s Heard on the Street column which discusses the efforts of Walmart and Amazon to enter the healthcare business.