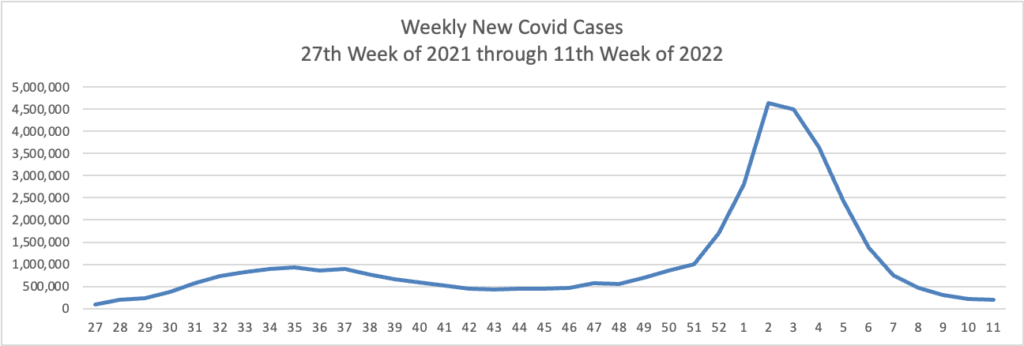
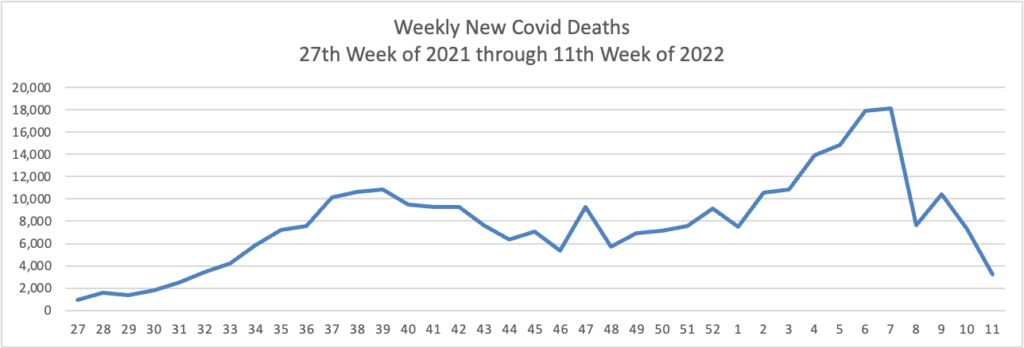
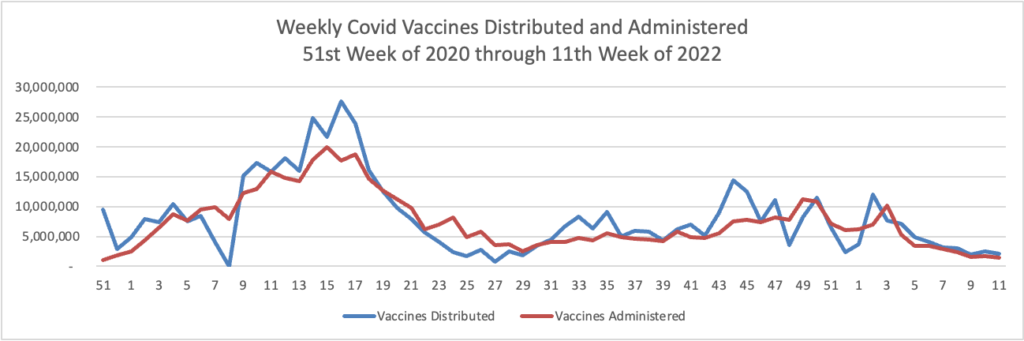
Weekend Update

Today has been the first day of Spring. But, it is the first day of Fall for my youngest son, who is studying medicine at Queensland University in Brisbane, Australia.
The House of Representatives is on a district work break this week. Meanwhile, the Senate will be engaged in committee business and floor voting on Capitol Hill.
Fedweek discusses the highlights of the call letter for 2023 benefit and rate proposal letters that OPM released last week.
OPM has told FEHB carriers to continue with increased levels of telehealth and other services related to the pandemic in the 2023 plan year, while also either ordering or encouraging them to expand benefits for certain other conditions.
While Fedweek gets the facts right, its article overlooks that OPM’s orders diminish FEHB competition which Congress relies upon to control premiums.
From the cost management front, Fierce Healthcare tells us
While prior authorization is a key tool in an insurer’s arsenal as it thinks about managing costs, the process remains a key source of friction with physicians.
Amid a nationwide conversation around addressing physician burnout and stress, plans are seeking ways to grow automated and virtual prior auth technologies to ease those barriers. At eviCore Healthcare, the benefit management arm of Cigna’s Evernorth subsidiary, the team has found one of the keys to success in this endeavor is bringing physicians into the conversation early on.
Eric Gratias, M.D., eviCore’s chief medical officer, told Fierce Healthcare that prior authorization programs are often built by people who, while well-intentioned, lack first-hand knowledge of what a clinical encounter is like.
The company’s own prior authorization technology, intelliPath, is built on collaboration with physicians who are actively practicing, he said.
“That partnership with our provider clients is absolutely critical for our success,” Gratias said.
Good point.
From the Rx coverage front
- NPR Shots points out significant problems with the distribution of Covid treatments.
[D]ata on COVID treatment utilization, shared with NPR by the U.S. Department of Health and Human Services, indicates that millions of COVID treatments are sitting on shelves unused.
“We are still in a public health emergency,” said Dr. Derek Eisnor, who leads the government’s distribution of COVID drugs, on a call with national health organizations on March 16. He urged health leaders to try to get the drugs to communities that have a demand for them, rather than let them go to waste.
“There’s an assumption that there’s not enough of [these drugs] around but it does seem when you look at the numbers that there is a lot around — it’s just not being used,” says Dr. Amesh Adalja, an infectious disease physician and senior scholar at the Johns Hopkins Center for Health Security. “They clearly are not getting to people at high enough rates to have their maximum impact.” * * *
It can be hard to know which pharmacies have the pills in stock or which infusion clinics have appointments available. A patient needs to be able to quickly find a clinician, get a diagnosis and prescription, and be able to access the treatment, all within a few days.
“It’s multifactorial why these drugs are underutilized,” Adalja says. “It’s likely all of those things are playing some role in the discrepancies between what’s been ordered and and what’s actually been administered.”
The Biden administration launched the Test to Treat initiative this month to address these gaps. “We know the challenges that are involved with patients obtaining therapeutics,” said Dr. Meg Sullivan, acting chief medical officer for HHS’ Office of the Assistant Secretary for Preparedness and Response, in a call with clinicians on March 12. The program aims to improve access to rapid testing and to bolster public and provider awareness of available COVID treatments and how to get them.
Here is a link to the HHS website that offers a U.S map that “displays public locations that have received shipments of U.S. Government-procured COVID-19 therapeutics under U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) authority. The long-acting antibody combination, Evusheld; monoclonal antibody treatments, bebtelovimab and sotrovimab; as well as the oral antiviral therapies, Paxlovid and molnupiravir are products authorized by the FDA for either prevention (Evusheld) or treatment (Paxlovid, sotrovimab, bebtelovimab, and molnupiravir) of COVID-19. The locations displayed in the locator have reported available courses within the last seven days.”
The test to treat program’s website is not online yet as far as the FEHBlog can tell.
- STAT News informs us that
On March 30, the Food and Drug Administration is bringing together outside experts in neurology to review an experimental drug from Amylyx for the treatment of amyotrophic lateral sclerosis, or ALS. The hearing is expected to be closely watched by ALS patients and their advocates, given the significant need for new treatments for the disease.
But the hearing is likely to garner extra attention because it’s the first meeting of the FDA advisory group since it met in November 2020 and voted unanimously against the approval of Aduhelm, Biogen’s drug for Alzheimer’s disease. The FDA later ignored that recommendation and approved the medication, leading to the resignation of three members of the panel and an uproar over whether the agency had compromised its standards.
Sixteen months removed from all the Aduhelm drama, the Peripheral and Central Nervous System (PCNS) Drugs Advisory Committee, restocked with new members, is back to tackle another high-stakes review of a drug targeting a progressive, fatal nervous system disease. The Amylyx treatment — called AMX0035 — isn’t likely to generate the same acrimony as Aduhelm, but it could similarly force the FDA to bend its standards.
Here’s what you need to know about AMX0035, the data from its single clinical trial, and the issues that are likely to take center stage at the FDA advisory panel meeting.