Thursday Miscellany

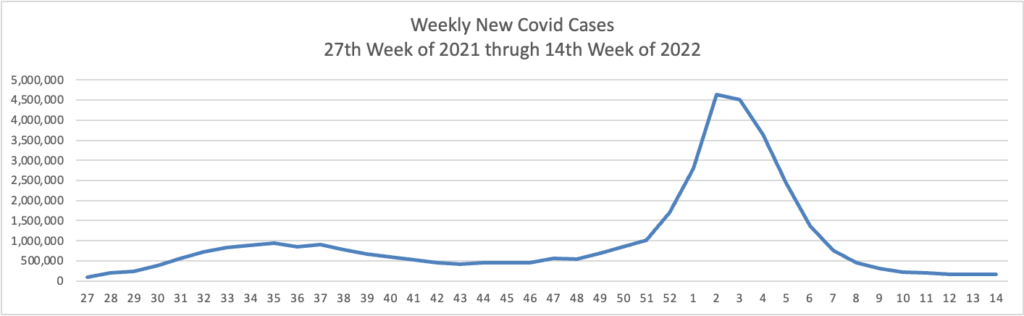
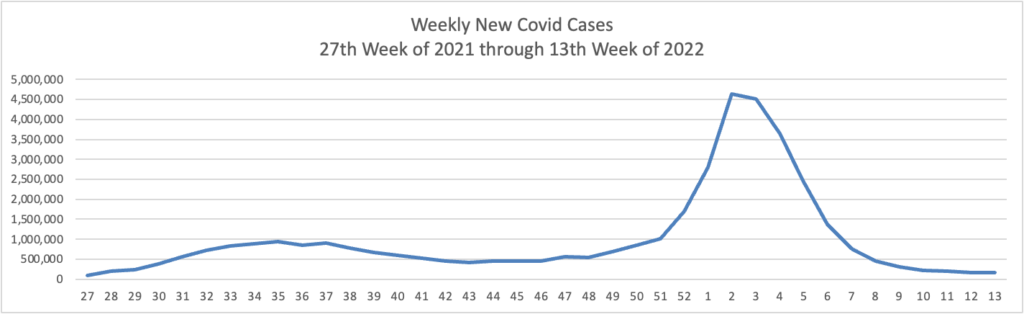
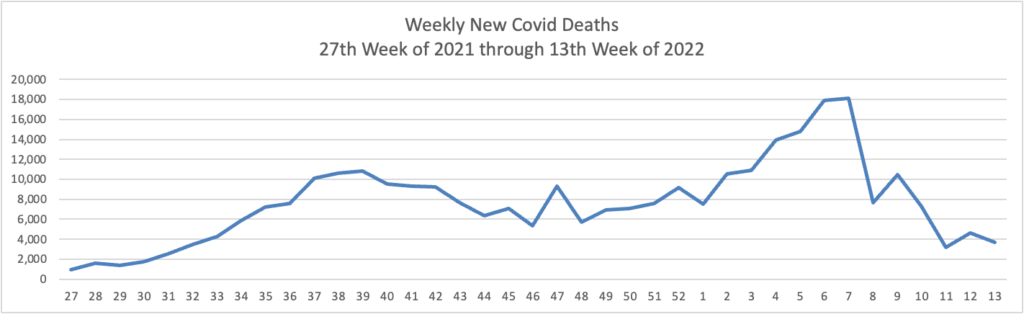
From the Omnicron and siblings front, the Wall Street Journal reports encouraging news.
The Omicron BA.2 variant has dominated new infections in the U.S. for weeks without setting off a major surge so far, raising hopes among some public-health experts that the nation might dodge a more significant hit.
BA.2 is in particular affecting the Northeast, where virus concentrations in wastewater are rising alongside reported infections in such places as New York, Washington, D.C., and Philadelphia. Concern about BA.2 prompted Philadelphia to restore an indoor-mask requirement and U.S. authorities to extend mask mandates for airplanes and other forms of transportation.
Still, BA.2 hasn’t yet caused the rise in hospitalizations some doctors said they would have anticipated. Disease experts say some combination of immunity from Covid-19 vaccinations and a severe wintertime surge, aided by springtime weather drawing people outdoors, might be keeping the virus at bay.
MedPage Today informs us
A booster dose of the Pfizer-BioNTech COVID-19 vaccine was safe and produced an immune response in kids ages 5 to 11, including against the Omicron variant, the companies said on Thursday.
These data came from two sources: the phase II/III clinical trial on 140 children ages 5 to 11 who received a booster dose at least 6 months after their two-dose primary series, and a subgroup of 30 kids in whom response against Omicron was studied specifically. In this subgroup analysis, there was a 36-fold increase in neutralizing antibody titers compared with levels seen after the two-dose primary series, the companies reported.
The companies plan to submit a request to the FDA for an emergency use authorization (EUA) for a third dose for this age group “in the coming days.” The agency previously authorized a two-dose primary series of the 10 μg formula for this age group in October 2021.
Health IT Analytics tells us, “When comparing groups that experienced the worst effects of COVID-19, a study published in Public Health Nursing found that the pandemic had a significant impact on those who exhibit high social vulnerability, leading them to have the highest mortality levels.” This finding illustrates the importance of resolving health disparities.
On a related note, Govexec reports
More than 90 federal agencies released their first-ever equity action plans on Thursday, laying out more than 300 strategies to better help underserved communities. This follows an executive order President Biden issued on day one of his administration, which directed agencies to conduct equity assessments of their top three to five high-impact services for Americans to determine where there were systematic barriers. These findings helped agencies develop their plans.
“Taken together these 300 actions demonstrate what it means to take a whole-of-government approach to advancing equity,” said a senior administration official on a background briefing call. “For the first time Americans will see a full picture of what it looks like for the entire federal government to advance equity at once.”
For example, Health and Human Services Department plans to better help individuals with limited English proficiency access federal health programs; the General Services Administration seeks to assess the impact on communities of its vast real estate portfolio; and the Office of Personnel Management looks to invest in data to look at potential barriers in the federal hiring process.
In a significant development from the No Surprises Act front, the Affordable Care Act regulators issued helpful Federal Independent Dispute Resolution (IDR) Process Guidance for Disputing Parties and Certified IDR Entities. The new guidance no longer treats the Qualifying Payment Amount as a rebuttable presumption. This action strongly suggests that the QPA’s rebuttable presumption status will be removed from the “final, final” version of the IDR rule. That regulation is due out next month. However, the rule does not yet appear on the OMB Office of Information and Regulatory Affairs’ list of rules currently being subjected to their oversight.
In other regulatory news, the International Foundation of Employee Benefits Plans alerts us,
The Department of Justice (DOJ) released guidance including frequently asked questions (FAQs) on how the Americans with Disabilities Act (ADA) protects individuals from discrimination when they are being treated for and recovering from opioid use disorder (OUD).
From Capitol Hill, EndPoint News reports
A group of 30 bipartisan lawmakers sent letters to 7 naloxone manufacturers, calling on them to apply for over-the-counter status for their opioid overdose antidotes and open up supplies further as the opioid crisis continues in the US with record levels of overdoses and deaths.
Citing a Massachusetts study that found substantially increased access to naloxone reduced opioid overdose mortality rates by 46%, the senators and representatives called on Pfizer, Teva Pharmaceuticals, Adamis Pharmaceuticals, Akorn, Amphastar Pharmaceuticals, Emergent BioSolutions, and Hikma Pharmaceuticals to “submit applications to make naloxone available over the counter without delay.”
Currently, there are three FDA-approved forms of naloxone — injectable, auto-injector and nasal spray — and all three currently require a prescription, but in most states and the District of Columbia pharmacists are allowed to dispense naloxone under a standing order, meaning they don’t actually need individual prescriptions. Some states also have given pharmacists direct authority to prescribe and sell naloxone to consumers.
Good idea. The HHS Secretary Xavier Becerra extended the opioid epidemic public health emergency for another 90 days earlier this month.
From the healthcare business front, Healthcare Dive reports on UnitedHealth Group’s 1st Quarter 2022 financial results.
UnitedHealth is bullish on completing its controversial acquisition of data analytics firm Change Healthcare, despite legal action from the Department of Justice to block the deal.
UnitedHealth’s extended agreement with Change “reflects our firm belief in the potential benefits of this combination to improve healthcare and in our ability to successfully overcome the challenge to this merger,” Chief Operating Officer Dirk McMahon told investors on a Thursday morning call regarding first-quarter financial results.
The Minnetonka, Minnesota-based healthcare behemoth beat Wall Street expectations for earnings and revenue in the quarter, with a topline of $80.1 billion, up 14% year over year due to double-digit growth at health services arm Optum and payer business UnitedHealthcare. Net earnings were $5.1 billion, up 3% year over year. UnitedHealth raised its full-year guidance following the results.
STAT News adds
The Omicron surge didn’t lead to an explosion of medical claims at UnitedHealth Group, which contributed to higher-than-expect profits. UnitedHealth ended the first three months of the year with more than $5 billion of profit on $80.1 billion of revenue. The company’s medical loss ratio, which shows the percentage of insurance premiums that were spent on medical claims, was 82% — higher than 80.9% in the first quarter of 2021, but less than what Wall Street expected.
From the miscellany department
- The ICD 10 Monitor discusses “two extremely encouraging studies in terms of the content coverage and feasibility of replacing ICD-10-CM with ICD-11.”
- BioPharma Dive reports “AbbVie and Genmab said treatment with their dual-acting antibody epcoritamab led to responses in nearly two-thirds of patients with lymphoma, announcing on Wednesday that their clinical trialsurpassed its benchmark for success. The partners will now take the data to the Food and Drug Administration and other regulators to determine whether it’s good enough to formally submit for approval.”
- Health Data Management offers useful insights into the ongoing TEFCA launch.