Friday Stats and More
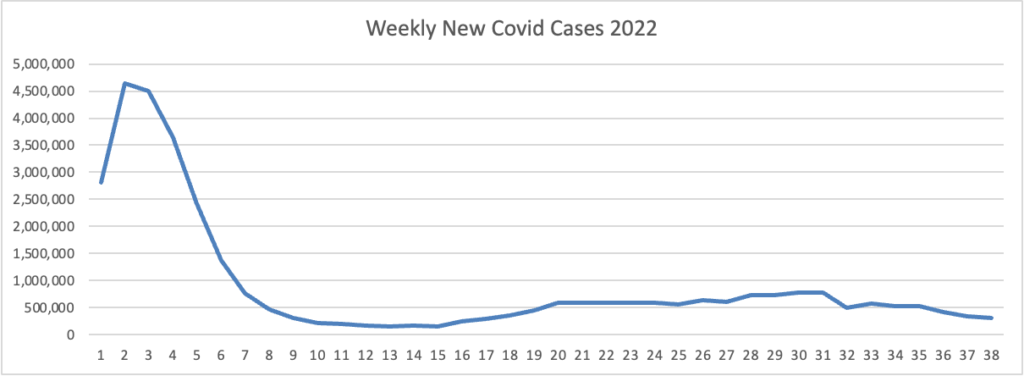
Based on the Centers for Disease Control’s Covid Data Tracker and using Thursday as the first day of the week, here is the FEHBlog’s latest weekly chart of new Covid cases.
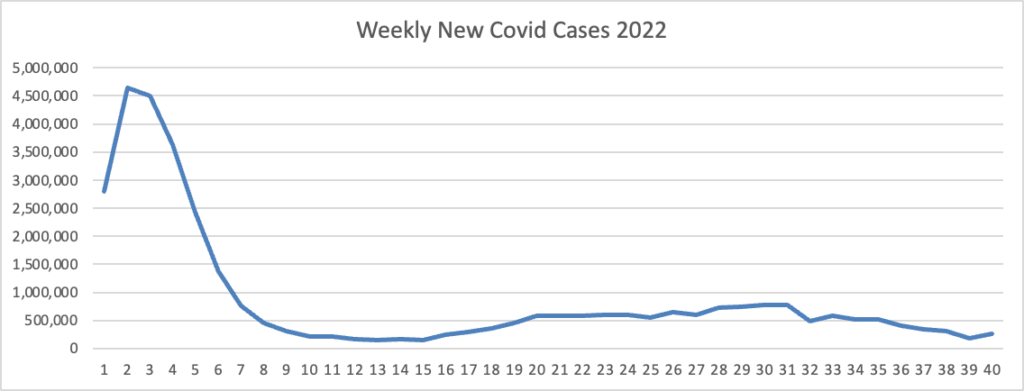
The CDC’s Covid Data Tracker Weekly Review was not issued today because Monday is a federal holiday.
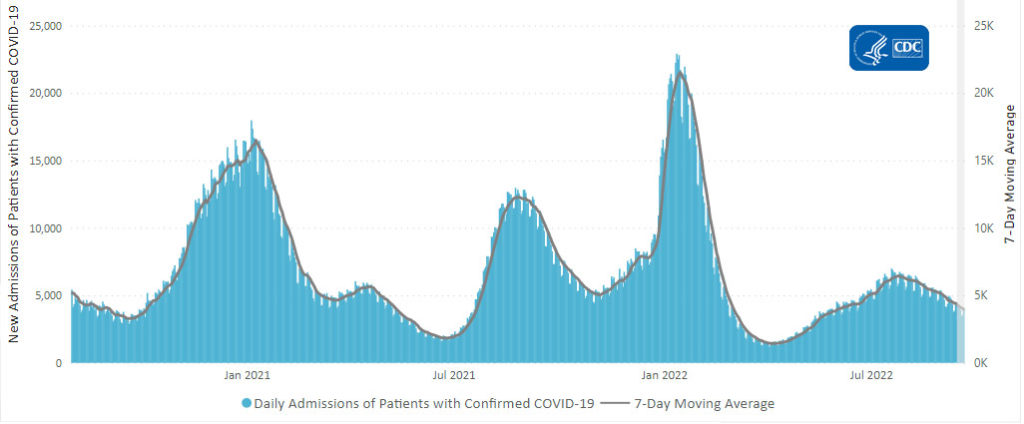
The Covid Weekly Tracker tells us that the daily average of new Covid hospital admissions is 3,35.
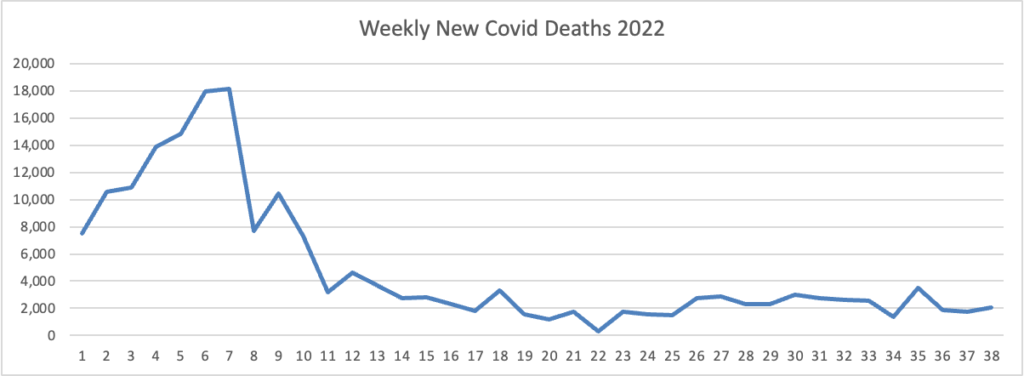
Here is the FEHBlog’s latest weekly chart of new Covid deaths.
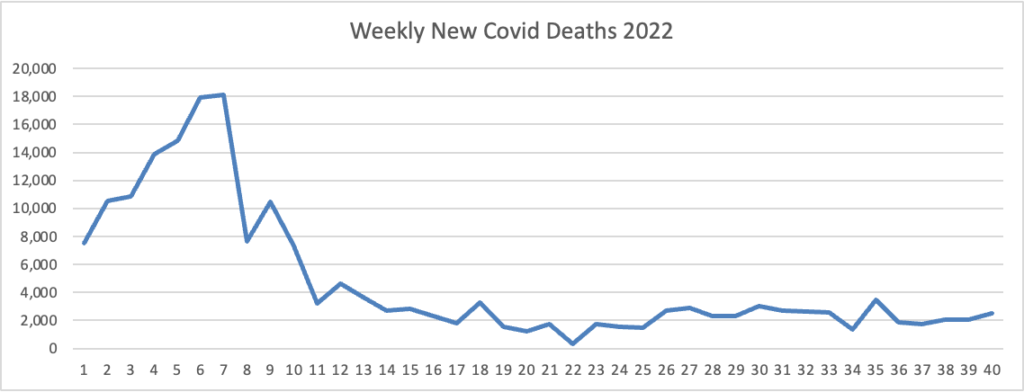
New York Times columnist David Leonhardt, who is going on a book tour that ends in late January 2023, reports
“A large chunk of deaths are preventable right now with Paxlovid alone,” Dr. Ashish Jha, the White House Covid response coordinator, told me. He predicted that if every American 50 and above with Covid received a course of either Paxlovid or a treatment known as monoclonal antibodies, daily deaths might fall to about 50 per day, from about 400 per day in recent months. * * *
A recent analysis of about 568,000 patients by Epic Research found that 0.016 percent of Covid patients over 50 who received Paxlovid died. The death rate for patients who did not get the drug was more than four times higher, or 0.070 percent. And yet the Epic data showed that only about 25 percent of patients eligible to receive Paxlovid actually did, even though the drug is widely available and free for patients.
Perhaps the most shocking statistic about Paxlovid’s underuse — and Jha used the word “shocking” when describing it to me — is that a smaller share of 80-year-olds with Covid in the U.S. is now receiving the drug than 45-year-olds with Covid, according to data he has seen. Many doctors are evidently worried about side effects or rebound cases among their more vulnerable patients.
Even in rebound cases, however, symptoms tend to be milder than they would have been without Paxlovid. After Dr. Anthony Fauci, another White House adviser, who’s 81, contracted Covid in June and then took Paxlovid, he experienced a rebound — and also believed that the drug kept him out of the hospital.
“Medicine is about weighing costs and benefits,” Wachter said. “The recommendation should be clear and unambiguous for people at high risk: The benefits of the drug outweigh the downsides.”
In contrast, STAT News reports
A Merck pill used to combat Covid-19 failed to demonstrate it can lower the risk of hospitalization compared with a placebo among adults at a higher risk from the disease, according to the results of a large study conducted in the U.K.
The preliminary results of the randomized trial, which involved more than 25,000 participants, showed that taking molnupiravir did speed time to recovery by about six days, which means that patients did get some relief. Otherwise, though, the study failed to reach an outcome that had been used late last year by regulators — such as those in the U.S. and U.K. — to authorize the medicine to thwart the pandemic.
The findings also contradict the results of a much smaller study conducted by Merck and its partner, Ridgeback Therapeutics, which found a lower risk of hospitalization or death in high-risk patients by roughly 30%, after initially showing a 50% lower risk. Unlike the latest trial, which is called Panoramic, the Merck trial called Move-Out excluded patients who had been vaccinated against the coronavirus.
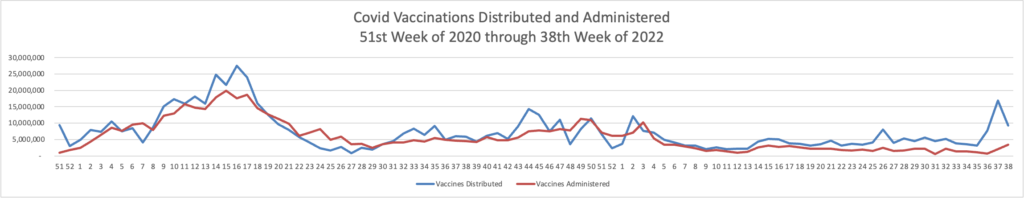
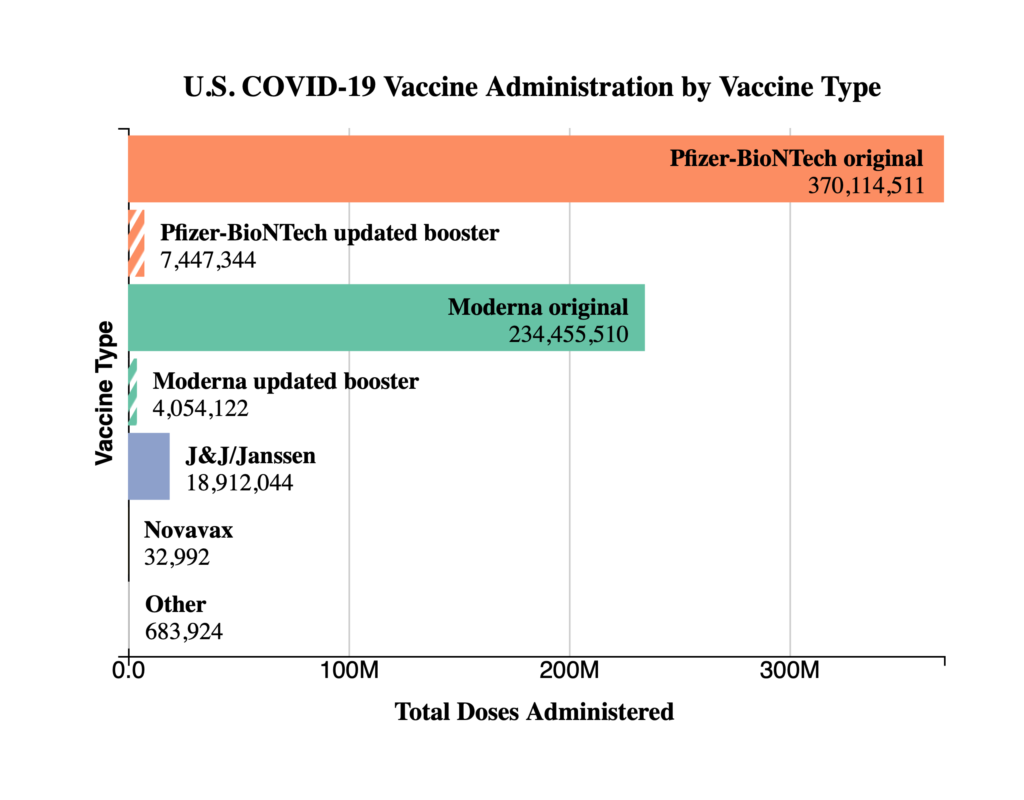
Here is the FEHBlog’s chart of Covid vaccinations distributed and administered from the beginning of the Covid vaccination era in December 2020 through the 40th week of 2022:
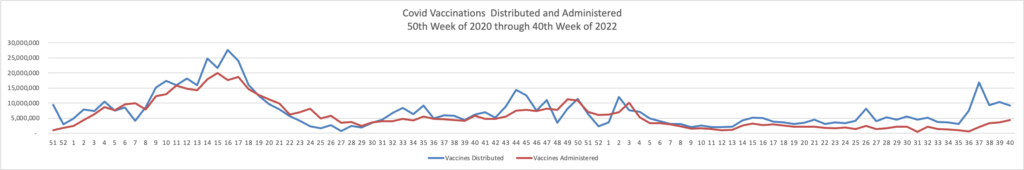
In addition, here are two related CDC charts.
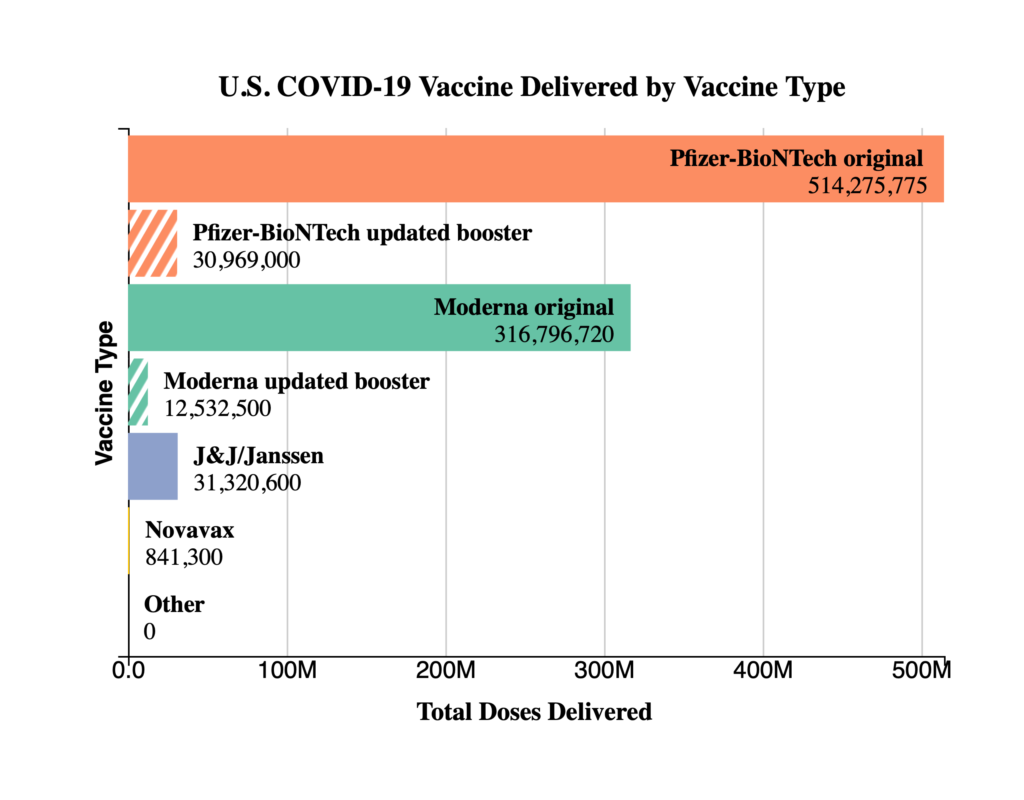
The American Hospital Association adds
COVID-19 vaccinations are associated with over 650,000 fewer hospitalizations and 300,000 fewer deaths in the Medicare population through December 2021, saving an estimated $16 billion in direct medical costs, the Department of Health and Human Services reported today.
“This report reaffirms what we have said all along: COVID-19 vaccines save lives and prevent hospitalizations,” said HHS Secretary Xavier Becerra. “We now have updated COVID vaccines designed to protect you against the Omicron strain of COVID that makes up almost all COVID cases in the U.S. … Over 90 percent of Americans live within 5 miles of where they can access these vaccines for free. I urge everyone eligible to get an updated COVID vaccine to protect yourself ahead of the fall and winter.”
Govexec tells us The Office of Personnel Management on Thursday announced that it authorized paid leave for federal workers to obtain the latest round of boosters for the COVID-19 vaccine.
From the FEHB front, Health Payer Intelligence reviews 2023 Blue Cross FEP benefit changes and makes other Open Season observations.
In OPM news, the GSA announced that its Technology Modernization Fund will be investing in OPM’s website.
OPM.gov Modernization
It can be challenging for federal employees, job seekers, and HR professionals to navigate OPM.gov’s 20,000 pages to find what they need. With a $6 million TMF investment, OPM will update both the technology behind and the content on the OPM.gov website. This will allow OPM to implement an updated and more secure Content Management System (CMS) hosted on OPM’s cloud environment, ensuring that users have intuitive and accessible web tools.
“A user-friendly website plays a critical role in OPM’s mission to communicate the federal government’s policies, services, and benefits more clearly and effectively,” said OPM Director Kiran Ahuja. “This investment will improve the government’s ability to recruit job seekers, supply the federal workforce with relevant career-related information, and make it easier for public servants to manage their benefits.”
Hope springs eternal.
From the mental healthcare front, Fierce Healthcare informs us
A mental health crisis besets young adults in the United States to such an extent that more than a third (35%) of individuals ages 18 through 29 years old said that they could not work nor engage in other activities of daily living, according to a new survey by the Kaiser Family Foundation (KFF) and CNN.
Meanwhile, 90% of all Americans believe that the country faces a mental health crisis.
Age 30 seems to be the cutoff separating severe crisis caused by mental health problems, and conditions not as dire. For instance, 34% of those 18 through 29 consider their mental health to be “only fair” or “poor”; 19% of those 30 and over feel that way. Fifty-two percent of young adults said that they’d always or often felt anxious in the last year, while 28% of older adults felt that way.
A third of young adults felt depressed (33%) or lonely (32%) in the last year; for older adults it was 18% for both depressed and lonely.
These survey figures, particularly the first one, are hard to believe, but undoubtedly our country needs more mental health therapists and better treatments.
From the Rx coverage front, the Food and Drug Administration announced that the agency has
approved Boostrix (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed [Tdap]) for immunization during the third trimester of pregnancy to prevent pertussis, commonly known as whooping cough, in infants younger than two months of age.
“Pertussis disease is a highly contagious respiratory illness affecting all age groups. However, babies are at highest risk for getting pertussis and having serious complications from it,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “While vaccination is the best method for providing protection, infants younger than two months of age are too young to be protected by the childhood pertussis vaccine series. This is the first vaccine approved specifically for use during pregnancy to prevent a disease in young infants whose mothers are vaccinated during pregnancy.”
Pertussis is a common respiratory disease in the United States, resulting in frequent outbreaks. It is also called whooping cough because of the “whooping” sound that someone makes when gasping for air after a fit of coughing. Most serious pertussis cases, hospitalizations and deaths occur in infants younger than two months of age who are too young to be protected by the childhood pertussis vaccine series. According to the Centers for Disease Control and Prevention (CDC), 4.2% of the total cases of pertussis reported in the United States in 2021 were in infants younger than 6 months of age and approximately 31% required hospitalization. When the Boostrix vaccine is given during pregnancy, it boosts antibodies in the mother, which are transferred to the developing baby.
Good news.
From the healthcare business front, Fierce Healthcare reports
Yale New Haven Health has signed an agreement to acquire two Connecticut health systems, Waterbury HEALTH and Eastern Connecticut Health Network), from Prospect Medical Holdings.
The deal would give Connecticut’s largest health system the businesses, real estate, physician clinic operations and outpatient services of three hospitals: 357-bed Waterbury Hospital, 249-bed Manchester Memorial Hospital and 102-bed Rockville General Hospital. Also included are Prospect Provider Group of Connecticut and Visiting Nurse and Health Services of Connecticut, according to a release.