Friday Factoids

From the Omicron and siblings front —
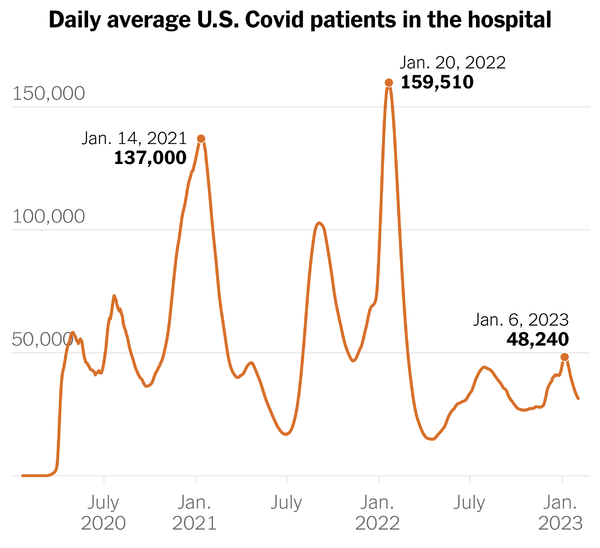
The CDC’s Covid Data Tracker shows all news cases, hospitalizations and deaths statistics trending down.
Health Day informs us, “Natural immunity from a COVID infection confers protection on par with that from mRNA vaccines, but patients run the risk of hospitalization and death during their initial infection.”
“The vaccines and boosters are still the safest way to acquire immunity, mainly for those who are in high-risk populations,” said study co-author Caroline Stein, a postdoctoral researcher at the University of Washington’s Institute for Health Metrics and Evaluation.
In fact, the best protection now appears to come from “hybrid immunity,” the combination of an actual infection and vaccination, said Dr. William Schaffner, medical director of the Bethesda, Md.-based National Foundation for Infectious Diseases.
From the public health front —
- The CDC’s weekly Fluview reports, “Seasonal influenza activity is low nationally.”
- The Fluview also discusses the risk that avian flu H5N1 poses to humans.
- “The current public health threat to people from the H5N1 virus is low. The current H5N1 outbreak in poultry and birds continues to be mostly an animal health issue. However, people should avoid direct and close contact with sick or dead wild birds, poultry, and wild animals. People should not consume uncooked or undercooked poultry or poultry products, including raw eggs. Consuming properly cooked poultry, poultry products, and eggs is safe. Other preventive measures are available at Bird Flu: Current Situation Summary “
- Health Day tells us, based on a recent CDC study, that “Far too many young kids aren’t eating enough fruits and vegetables, even as they consume plenty of sugary sodas.” No bueno.
- The Wall Street Journal considers “How Common is Depression After a Stroke? Sen. John Fetterman’s Treatment Prompts Question. Depression can often follow a major medical issue or chronic illness. Here’s what to know.”
- “Overall, between 20% and 30% of people with a chronic illness experience depression, says Karina Davidson, a clinical psychologist and dean of academic affairs at Northwell Health’s Feinstein Institutes for Medical Research.”
- The Mercer consulting firm explains how new approaches are filling gaps created by obstetric unit closings.
- “As the access to obstetrics care dwindles, employers can and should be looking for additional solutions to help keep their pregnant members and their newborns healthy. Whether that’s exploring new digital tools [such as Pregnascan and HeraMED] coming to market or just expanding coverage on their health plans to include doulas, community doulas, midwives, and birthing centers, any effort will be an important step forward in the fight to reduce maternal mortality and poor health outcomes in the US.”
From the No Surprises Act front, a colleague reminded the FEHBlog today that the deadline for health plans and air ambulance providers to submit their first NSA air ambulance report, March 31, 2023, is closing in. While the federal government has made available draft information on preparing this report, no sign of an official submission portal has appeared online. The clock continues clicking.
From the Miscellany Department —
- Medscape offers an excellent article on the cost of gene therapies. Remember to click pages.
- Mercer also delves into the abortion pill coverage quandary. (While FEHB plans must cover contraceptive pills, including the Plan B pill, abortion pills are excluded from coverage unless the mother’s life would be endangered if the fetus were carried to term or when the pregnancy is the result of an act of rape or incest.”
- The American Hospital Association puts a favorable spin on a recent CMS report on health system compliance with the federal government’s hospital pricing transparency rule.
- CMS is pleased “to provide you with the recording (password: 4%2M!3c?) and slides of the February 9, 2023, virtual education session hosted by the CMS Office of Burden Reduction and Health Informatics on the Advancing Interoperability and Improving Prior Authorization Processes proposed rule.
- As a reminder, we welcome your feedback on the proposed policies introduced in the CMS Advancing Interoperability and Improving Prior Authorization Processes proposed rule (CMS-0057-P). Comments must be received within the 90-day comment period, which closes on March 13, 2023. When commenting, please refer to file code: CMS-0057-P.”
- Beckers Hospital Review reports, “The Federal Trade Commission has welcomed the State University of New York Upstate Medical University and Crouse Health System’s decision to abandon their proposed merger, which it said would have left Syracuse with just two hospital systems — Upstate and St. Joseph’s Health — and given the combined entity a 67 percent share of commercially insured inpatient services in Onondaga County.”