Tuesday’s Tidbits

From Capitol Hill, Congress.gov informs us that Congress has sent the Postal Reform Act of 2022 (HR 3076) to the President for his signature.
MedPage Today discusses a Congressional hearing on Medicare for All held today. Democrats (for) and Republicans (against) remain split.
The FEHBlog finds the President’s budget proposal useful for identifying new Administration FEHB priorities, several of which were identified in yesterday’s post. What’s more, FedWeek tells us
[T]he FEHB program would be among programs affected by a broader proposal regarding mental health service coverage in health insurance. It would require coverage of three primary visits and three behavior health visits without cost-sharing.
In the FEHBlog’s opinion, this idea would drive up premiums for no reason because federal and postal employees already are offered employee assistance programs that offer free counseling sessions. OPM needs to do a better job coordinating its various benefit programs.
Fierce Healthcare identifies four other healthcare items from the President’s budget proposal that should be watched.
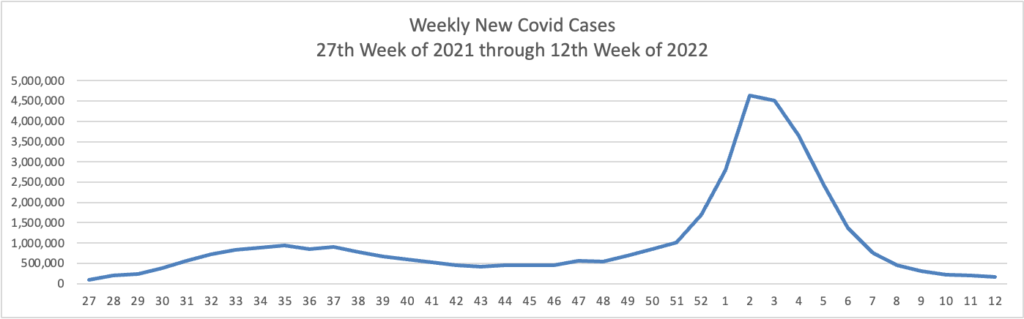
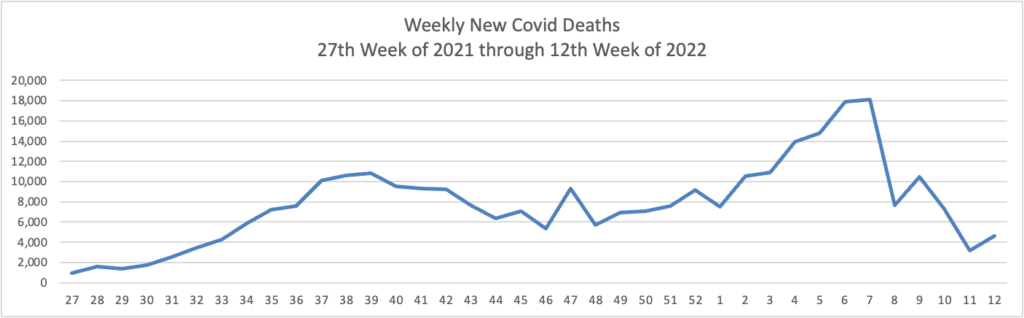
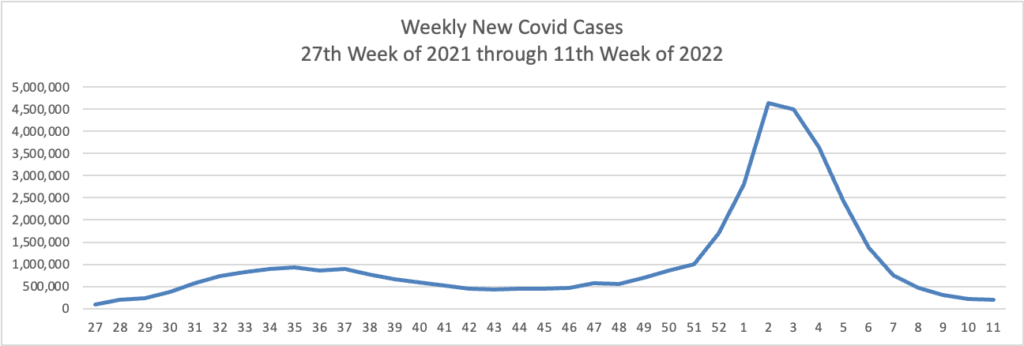
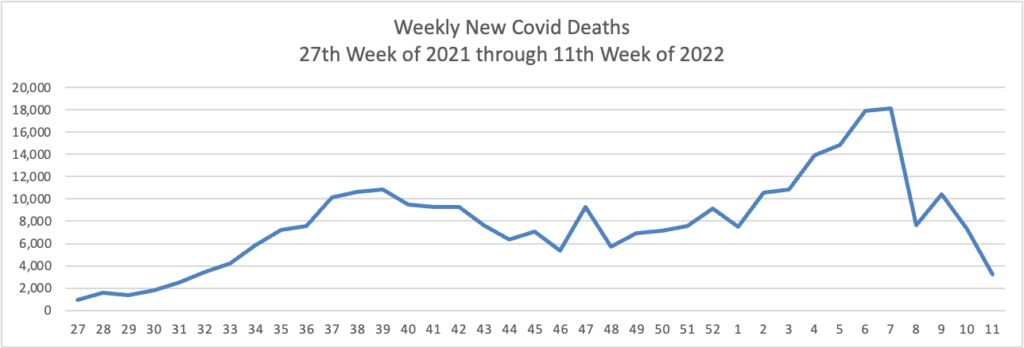
From the Omicron (and siblings) front —
The Wall Street Journal reports
The Omicron BA.2 variant represents more than half of new Covid-19 cases in the U.S., the latest federal estimates show, as signs suggest infections are edging higher again in parts of the Northeast.
The region has the highest BA.2 concentrations, including more than 70% in an area including New York and New Jersey, according to estimates the Centers for Disease Control and Prevention released Tuesday. BA.2 has been moving steadily higher for more than a month and represents an estimated 55% of national cases in the week ended March 26, the CDC said. * * *
“Predictions are hard, but I am expecting that the U.S. will have a surge in at least some locations,” said Aubree Gordon, an associate professor of epidemiology at the University of Michigan School of Public Health.
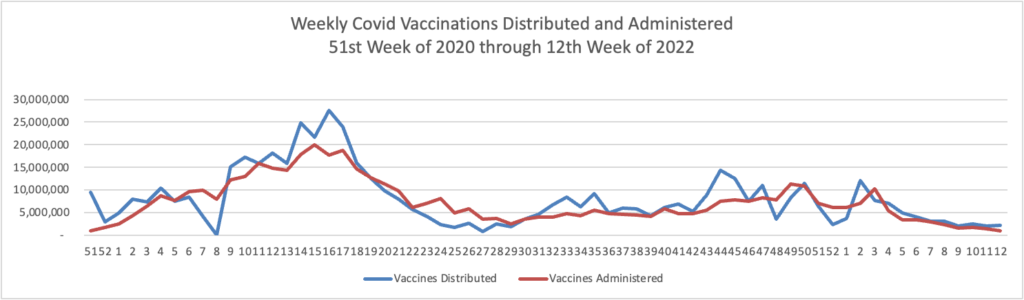
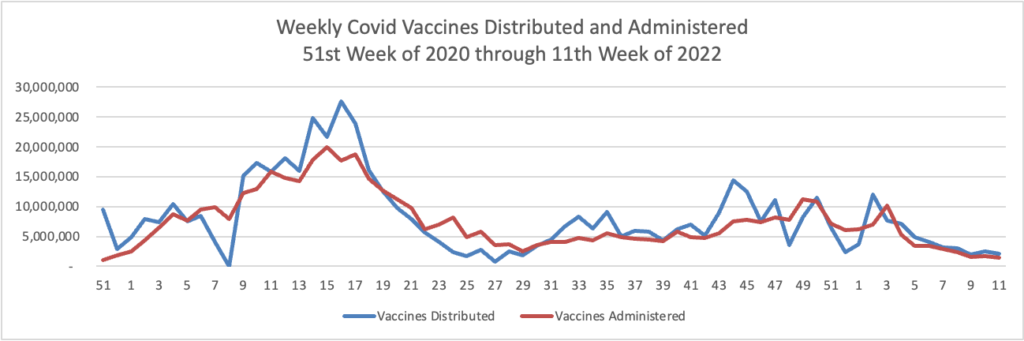
AHIP tells us
Today the Food and Drug Administration (FDA) authorized a second single Pfizer-BioNTech or Moderna COVID-19 vaccine booster dose for persons aged 50 and older at least 4 months after receipt of a first booster dose of any authorized or approved COVID-19 vaccine. A second booster dose of the Pfizer-BioNTech COVID-19 vaccine or Moderna COVID-19 vaccine may also be administered to certain immunocompromised individuals, for those 12 years of age and older or 18 years of age and older, respectively, at least 4 months after receipt of a first booster dose of any authorized or approved COVID-19 vaccine.
The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series. This action will now make a second booster dose of these vaccines available, for a total of five vaccine doses authorized for populations at higher risk for severe disease, hospitalization and death. Emerging evidence suggests that a second booster dose of an mRNA COVID-19 vaccine improves protection against severe COVID-19 and is not associated with new safety concerns. * * *
This authorization still requires the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) to formally recommend the vaccine for the specific populations. No date for an ACIP meeting has yet been announced.
Health plans are not required to reimburse these authorized vaccines until ACIP makes its decision.
From the healthcare business front —
Healthcare Dive reports
Hospitals’ operating margins were negative in February for the second consecutive month even as cases of the omicron variant waned, according to Kaufman Hall’s National Hospital Flash report. Negative margins in January were the first seen in 11 months.
The median Kaufman Hall Operating Margin Index was -3.45%, up from -4.25% in January but still well below levels hospitals can sustain, the report said.
Volumes for inpatient services fell while outpatient volumes staggered with revenues in those categories falling 19.3% and 5%, respectively, from January, according to the report.
Healthcare Finance News reports
UnitedHealth Group subsidiary Optum will combine with in-home healthcare service provider LHC Group, with UHG purchasing the latter for about $5.4 billion.
LHC provides healthcare services in the home for a demographic of mostly older patients dealing with chronic illnesses and injuries. It will be melded with Optum, which manages drug benefits and offers data analytics services and works with more than 100 health plans.
From the tidbits department —
- The CDC has posted a new, improved anti-biotic resistance website. The CDC explains that the site is “refreshed to better engage and share information on antibiotic resistance (AR) in the United States and around the world. We all have a role to play—from travelers, animal owners, and care givers to patients and healthcare providers—to fight this deadly threat and now you can quickly access CDC’s latest resources.”
- MedPage Today reports “Prediabetes prevalence nearly doubled among U.S. youth from 1999 to 2018, national data indicated. According to National Health and Nutrition Examination Survey (NHANES) data on over 6,500 youth, the prevalence of prediabetes increased from 11.6% in 1999-2002 to 28.2% in 2015-2018, Junxiu Liu, PhD, of Icahn School of Medicine at Mount Sinai in New York City, and colleagues reported in JAMA Pediatrics.” Obesity is a common thread.
- The Endocrinology Network informs us “Participation in a tele-mentoring program led by Robert Wood Johnson Medical School was associated with a 44% decrease in inpatient admissions and a more than 60% decrease in inpatient spending among Medicaid patients with diabetes.” Bravo.
- The International Foundation of Employee Benefit Plans discusses evaluating high cost gene therapy financing programs.