Friday Stats and More
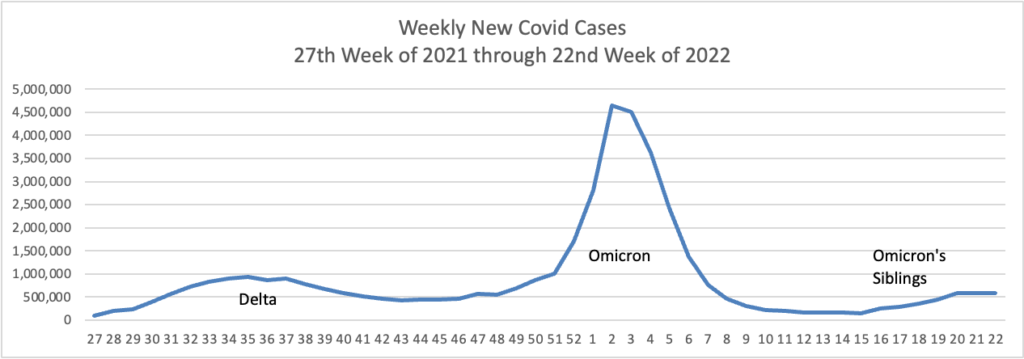
Based on the Centers for Disease Control’s Covid Data Tracker and using Thursday as the first day of the week are the FEHBlog’s weekly charts of new Covid cases for 2022:
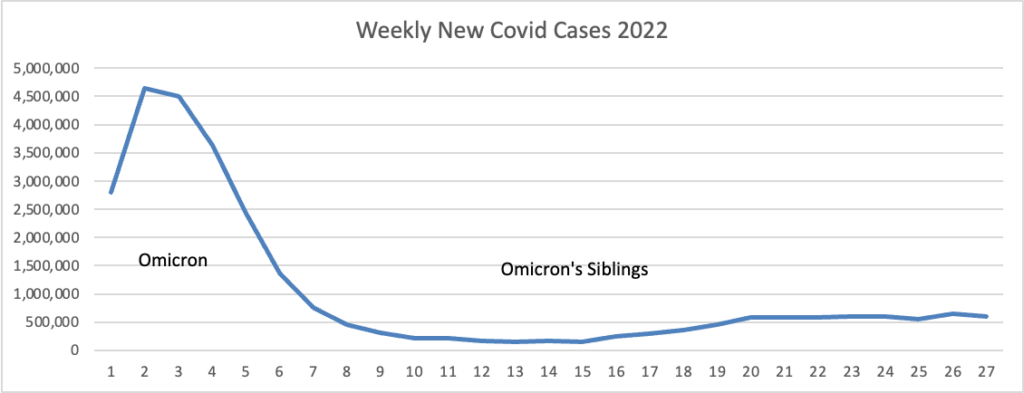
The CDC’s Weekly Review of its Covid Statistics observes “As of July 6, 2022, the current 7-day moving average of daily new cases (106,549) decreased 3.9% compared with the previous 7-day moving average (110,875).”
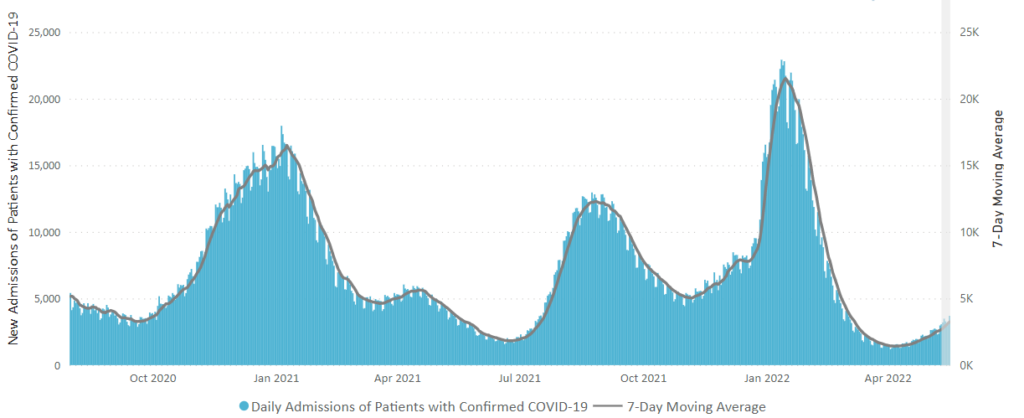
Here is the CDC’s weekly chart of new Covid hospital admissions
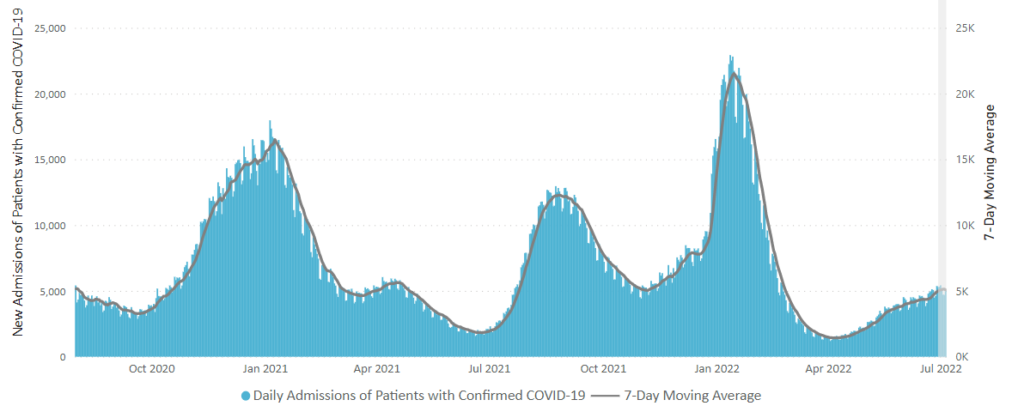
The CDC’s Weekly Review comments “The current 7-day daily average for June 29–July 5, 2022, was 5,080. This is a 3.1% increase from the prior 7-day average (4,930) from June 22–28, 2022.”
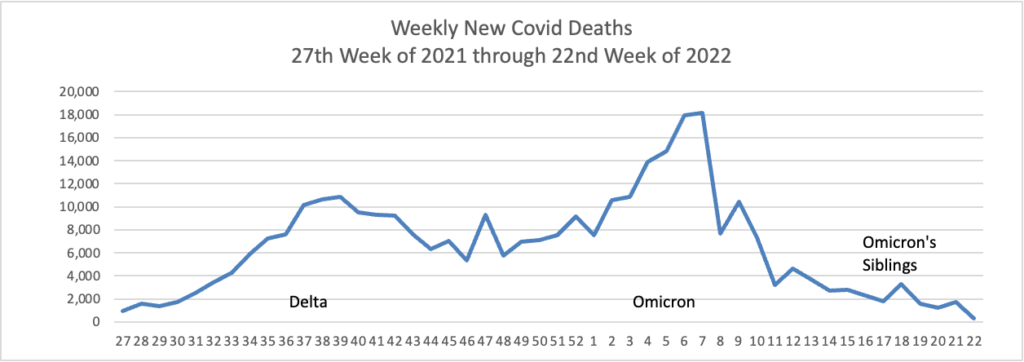
Here is the FEHBlog’s weekly chart of new Covid deaths for 2022
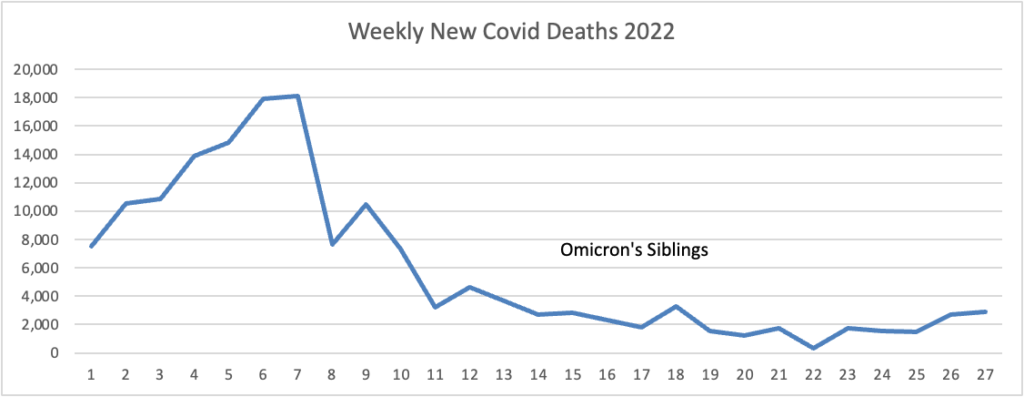
The CDC’s Weekly review observes “The current 7-day moving average of new deaths (273) has decreased 20.9% compared with the previous 7-day moving average (345).”
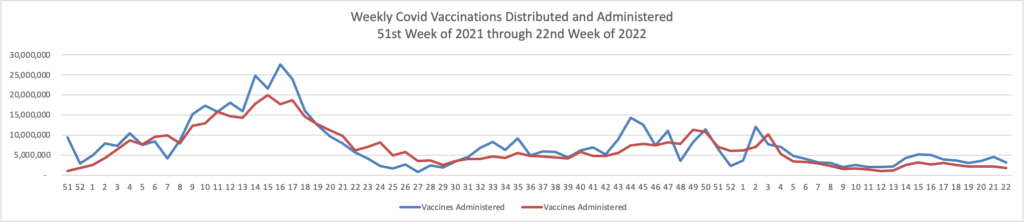
Here’s the FEHBlog’s weekly chart of Covid vaccinations distributed and administered from the beginning of the Covid vaccination era in the 51st week of 2020 through the 27th week of 2022.
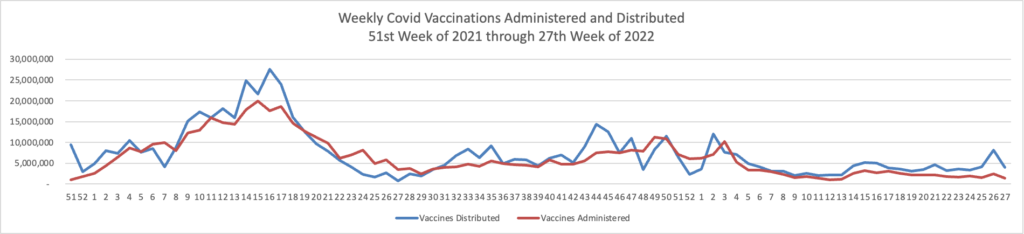
The CDC’s weekly review explains
Overall, about 260.3 million people, or 78.4% of the total U.S. population, have received at least one dose of vaccine. About 222.5 million people, or 67.0% of the total U.S. population, have been fully vaccinated.* Of those fully vaccinated, about 106.6 million people have received a booster dose,** but 50.1% of the total booster-eligible population has not yet received a booster dose.
The American Hospital Association adds
The Food and Drug Administration today granted full approval of Pfizer’s COVID-19 vaccine for young teens, covering the age group spanning 12 to 15 years old. FDA said the vaccine, which has been administered in two-dose regimens to nearly 9 million Americans, earned full approval following its “rigorous analysis and evaluation of the safety and effectiveness data.” The approval does not apply to booster doses for that age group.
The CDC’s Weekly Review also provides the following Communities news:
As of July 7, 2022, there are 666 (20.7%) counties, districts, or territories with a high COVID-19 Community Level, 1,218 (37.8%) counties with a medium Community Level, and 1,331 (41.3%) counties with a low Community Level. Compared to last week, this represents an increase (+1.3 percentage points) in the number of high-level counties, an increase (+2.4 percentage points) in the number of medium-level counties, and a corresponding decrease (−3.7 percentage points) in the number of low-level counties. 49 out of 52 jurisdictions* had high- or medium-level counties this week. Rhode Island, New Hampshire, and Washington, D.C., are the only jurisdictions to have all counties at low Community Levels.
To check your COVID-19 Community Level, visit COVID Data Tracker. To learn which prevention measures are recommended based on your COVID-19 Community Level, visit COVID-19 Community Level and COVID-19 Prevention.
From the Medicare front, Becker’s Hospital Review points out seven things to know about the CMS 2023 Medicare Part B physician payment rule issued yesterday. The lead item has grabbed the medical community’s attention.
The proposed physician fee schedule conversion factor for 2023 is $33.08, down from $34.61 in 2022. The proposal considers a statutory requirement that the conversion factor for 2023 remain flat as well, due to the expiration of the 3 percent increase in physician fee schedule reimbursement payments in 2022 that was required in the Protecting Medicare and American Farmers From Sequester Cuts Act.
For FEHB patients with primary Medicare Part B coverage, this reduction will amount to a cost shift from Medicare to FEHB if implemented by the final rule.
From the No Surprises Act front, Morning Consult offers the results of a survey finding that
One in 5 U.S. adults say they have received an unexpected medical bill this year, according to a new Morning Consult survey that underscores the prevalence of sticker shock in health care — even after federal efforts to combat it.”
The Morning Consult’s survey needs to be taken with a grain of salt because 22% of the surveyed adults are Medicare age (see Survey, p. 131), and the No Surprises Act does not apply to Medicare.
From the Rx coverage front, the PBM’s lobby PCMA compliments the Biden Administration for
taking real action to reduce prescription drug costs. Drug manufacturer pricing strategies that include patent thickets on certain brand drugs unfairly protect those drugs and biologics from generic and biosimilar competition.
The United States Patent and Trademark Office’s and the Food and Drug Administration’s enhanced review power for applications for drug patents that simply don’t deserve an intellectual property extension is a positive step toward eliminating patent thickets and bringing more competition to the marketplace.
That is thoughtful action by the PTO and FDA.
From the Dobbs front, the White House outlines an executive order the President plans to sign in response to the Supreme Court decision.
From the mental health front, Health Payer Intelligence discusses an initiative by “CVS Health and its payer arm, Aetna, [to] expand its existing program with Psych Hub, advancing its efforts around adolescent suicide prevention.” Bravo.
From the artificial intelligence front, the Wall Street Journal reports “Using advanced analytics and AI, health insurers are building targeted medical advice for customers. Now comes the hard part: Getting them to respond. ‘There’s an art to that data communication,’ one doctor says.” Fascinating read.