Monday Round-up

Yesterday, the FEHBlog discussed a U.S. Court of Appeals for the 11th Circuit opinion issued last Friday narrowing the scope of a nationwide injunction that a federal district court had imposed on the Biden Administration’s federal government contractor mandate.
Govexec adds today that
The executive order is still enjoined in Alabama, Alaska, Arizona, Arkansas, Florida, Georgia, Idaho, Indiana, Iowa, Kansas, Kentucky, Louisiana, Mississippi, Missouri, Montana, Nebraska, New Hampshire, North Dakota, Ohio, South Carolina, South Dakota, Tennessee, Utah, West Virginia and Wyoming as a result of the Friday opinion and the injunctions in the other cases, members of the law firm McGuireWoods noted in a post.
A spokesperson for the Office of Management and Budget told Government Executive on Monday morning the Justice Department is currently reviewing the decision. “At this time, the nationwide injunction remains in effect, and thus agencies should continue not to take any steps to enforce Executive Order 14042.”
The nationwide injunction remains in effect at least until the appellate court issues its mandate to the lower court which typically happens in two weeks.
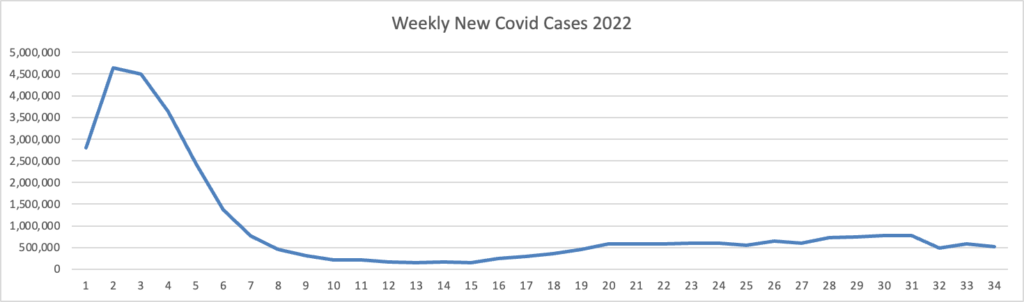
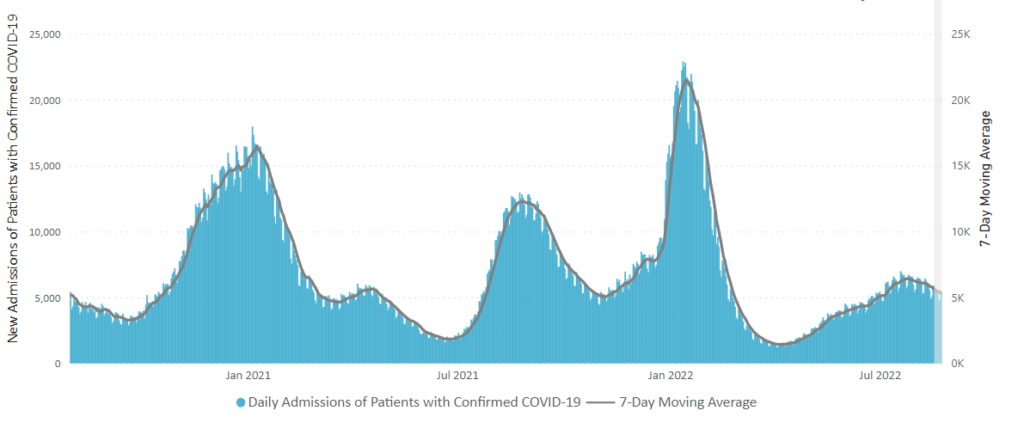
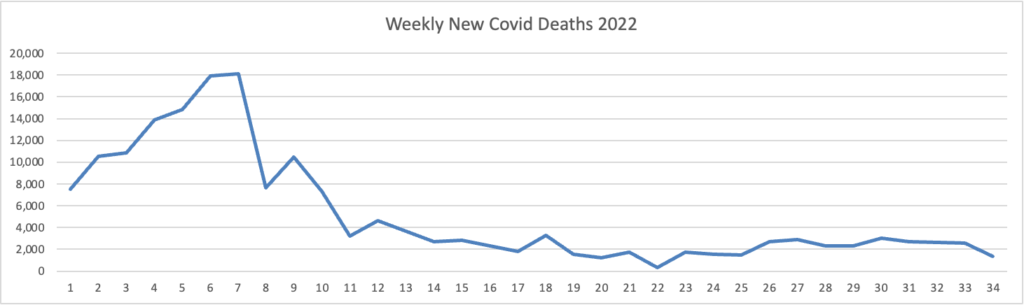
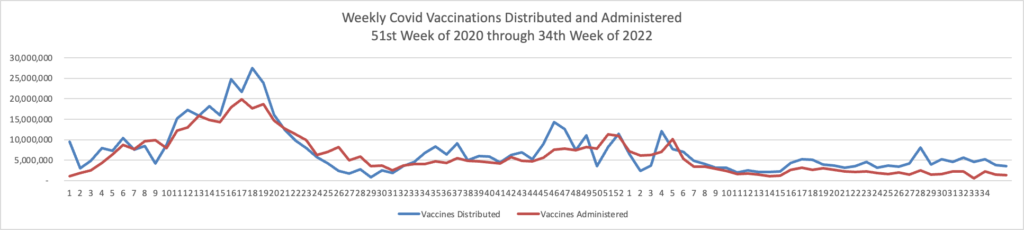
Also from the Omicron and siblings front, the Wall Street Journal discusses best practices for Covid testing while NPR tells us
The federal government is putting a pause on sending free COVID-19 testing kits to Americans starting in September, due to a lack of funding.
“Ordering through this program will be suspended on Friday, September 2 because Congress hasn’t provided additional funding to replenish the nation’s stockpile of tests,” the ordering website says.
However, the program is still accepting orders before [next Saturday] Sep. 2.
From the No Surprises Act front, Mercer Consulting announced
A new prescription drug reporting mandate, adopted as part of the 2021 Consolidated Appropriations Act (CAA) (Pub. L. No. 116-260), requires group health plans and health insurers to report detailed data about prescription drug pricing (including rebates) and healthcare spending. The first reports are due by Dec. 27, 2022, and annually thereafter. The departments of Labor, Treasury, and Health and Human Services will use the information to prepare a biannual, publicly available report. The departments have issued interim final rules (IFR) detailing the data to report and recently updated submission instructions describing the mechanics of the reporting process. The updated instructions provide important information about reporting wellness services, prescription drug expenses that are covered by the pharmacy benefit manager and more.
Download the 21-page print-friendly article for details on the prescription drug reporting rules and the compliance challenges facing group health plans. This GRIST has been updated to reflect the updated submission instructions.
OPM added FEHB plans to the list of reporting plans and insurers. The initial report on the 2020 plan year is due no later than December 27, 2022.
From the FEHB plan design front, Federal News Network reports
Democratic lawmakers are urging the Office of Personnel Management to follow through on its plans to expand federal employees’ medical coverage to cover infertility diagnoses and treatments.
Sen. Tammy Duckworth (D-Ill.) and Government Operations Subcommittee Chairman Gerry Connolly (D-Va.) urged OPM on Monday to ensure all Federal Employee Health Benefits (FEHB) program carriers provide coverage for assistive reproductive technology (ART), which includes in vitro fertilization (IVF), starting in 2023.
The legislators’ demand strikes the FEHBlog as a day late and a dollar short because FEHB carriers and OPM closed their benefit and rate negotiations earlier this month. OPM did ask carriers in the agency’s 2023 call letter to plan on expanding ART coverage or offering a discounted ART network, among other options.
From the maternal health front, the Department of Health and Human Services announced “investments of over $20 million to improve maternal and infant health and implement the White House Blueprint for Addressing the Maternal Health Crisis – PDF. Funding aims to help reduce disparities in maternal and birth outcomes, expand and diversify the workforce caring for pregnant and postpartum individuals, increase access to obstetrics care in rural communities, and support states in tackling inequities in maternal and infant health.”
From the mental healthcare front, Health Payer Intelligence delves into the resources that AHIP has made available.
Seven strategic themes emerged from the list of ways that payers have helped members manage their mental health needs:
* Helping members find the right providers
* Creating opportunities for care through telehealth, online platforms
* Designing new payment models
* Expanding and educating the mental healthcare workforce
* Offering population-based services
* Supporting caregivers
* Expanding research and awareness
The article expounds on each of these themes.
From the U.S. healthcare business front, Fierce Healthcare reports
Months of inching performance gains were upended in July as the nation’s hospitals logged “some of the worst margins since the beginning of the COVID-19 pandemic,” Kaufman Hall wrote in its latest industry report. * * *
What’s more, seven straight months of negative margins “reversed any gains hospitals saw this year” and has the advisory group forecasting a brutal year for the industry.
“July was a disappointing month for hospitals and put 2022 on pace to be the worst financial year hospitals have experienced in a long time,” Erik Swanson, senior vice president of data and analytics with Kaufman Hall, said in a statement. “Over the past few years, hospitals and health systems have been able to offset some financial hardship with federal support, but those funding sources have dried up, and hospitals’ bottom lines remain in the red.” * * *
The silver lining in Kaufman Hall’s report were total expenses that, although up 7.6% from July 2021, saw a modest 0.4% decline since June. Those savings came squarely among supply and drug expenses as total labor costs and labor expense per adjusted discharge still grew 0.8% and 3.5%, respectively, since June. Increases in full-time employees per adjusted occupied bed “possibly” suggest increased hiring, the group wrote in the report.
From the electronic health record interoperability front, Becker’s Health IT informs us
Judy Faulkner, CEO of Epic, discussed the company’s vision to build a nationwide health IT infrastructure last week at the annual Users Group Meeting while dressed as Amelia Earhart, according to The Cap Times.
Ms. Faulkner has a history of dressing as characters and historical figures for her highly anticipated keynote address at the meeting every year, and this year she chose Ms. Earhart, the iconic female pilot, as a nod to the meeting’s theme: A Night at the Museum. She talked about new technologies and expectations for Epic and its data platform, Cosmos. * * *
“We are building a nationwide health IT infrastructure to connect the different parts of healthcare,” Ms. Faulkner told the crowd.
Epic’s largest competitor in the hospital market, Oracle Cerner, is also on a mission to digitally connect the U.S. healthcare system. Larry Ellison, chair, co-founder and chief technology officer of Oracle, revealed in June the company’s plans to build a unified national healthcare database after acquiring Cerner earlier this year for $28.4 billion. His vision of a national healthcare database includes anonymized data from hospitals, clinics and providers to give real-time information about patients’ health as well as public health statistics.