Tuesday Tidbits

There are a boatload of tidbits today.
Roll Call reports that “The House [of Representatives] will vote to clear the $1.9 trillion pandemic relief package for President Joe Biden’s signature on Wednesday, Majority Leader Steny H. Hoyer told reporters.”
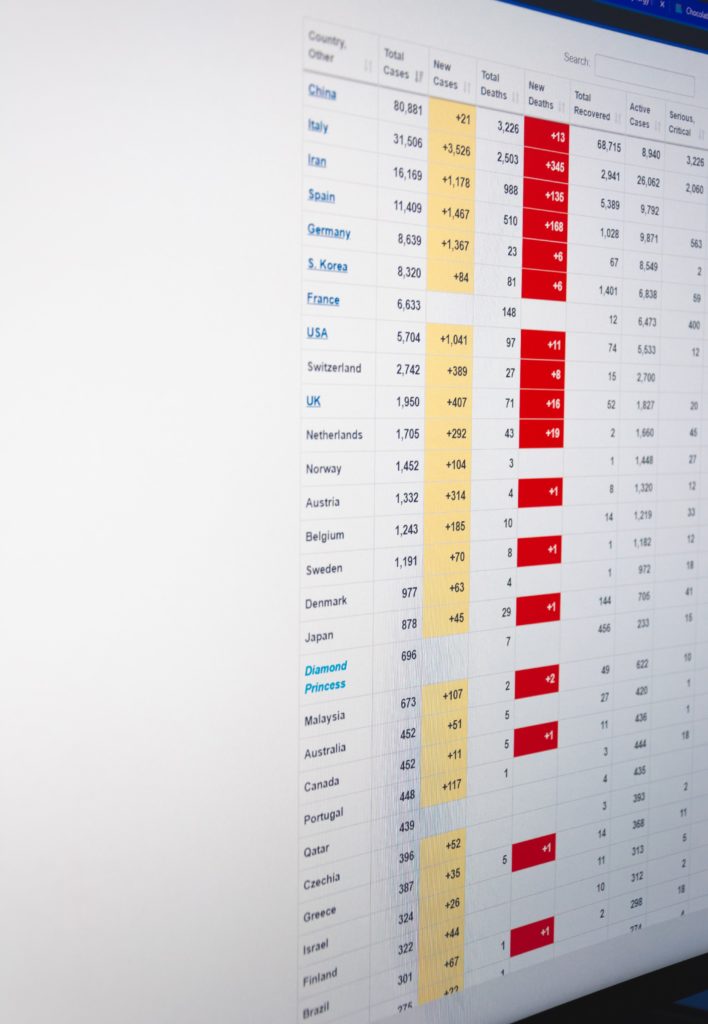
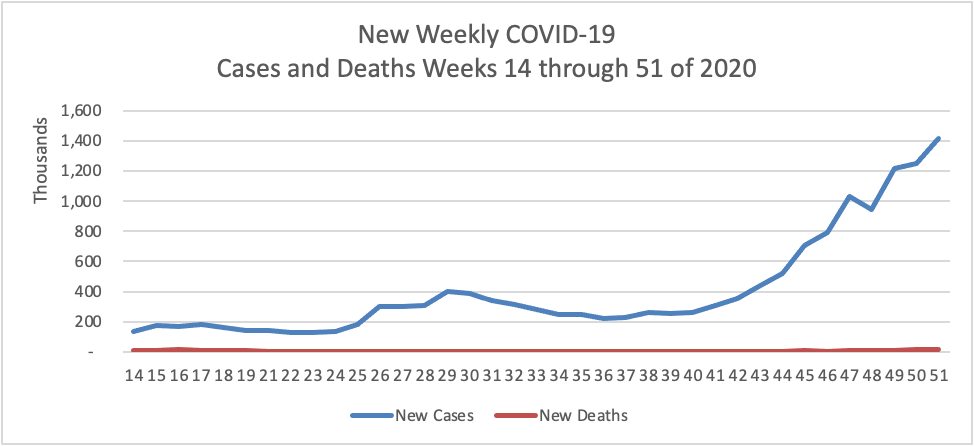
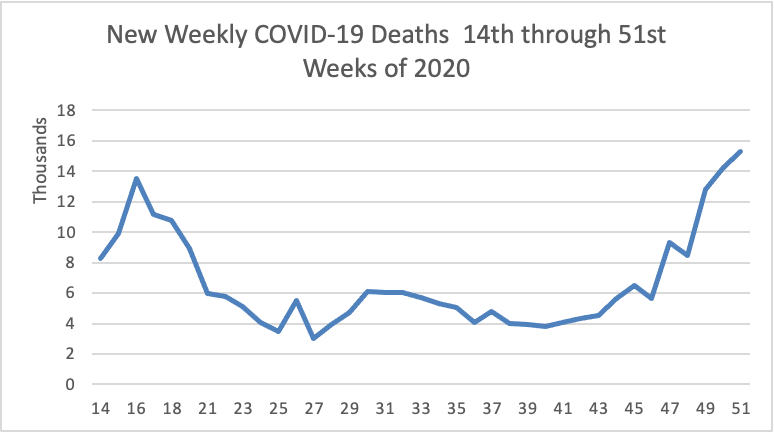
From the COVID-19 front
- Fierce Pharma informs us that “Vaccine doses are fanning out around the globe, but officials worry that surging coronavirus variants could make the immunization push less effective. Thanks to a new lab study, Pfizer and BioNTech have some good news for them. “Pfizer and BioNTech’s mRNA shot [which the FEHBlog has received] appeared to work against three worrisome variants in a lab study, researchers from both companies and the University of Texas Medical Branch wrote in the New England Journal of Medicine. That includes the P.1 variant that arose in Brazil and has raised concerns about re-infections.”
- Fierce Healthcare reports ” As the COVID-19 vaccine rollout continues, payers are gearing up to play a key role in easing vaccine fears and hesitancy. At Humana, for example, this has meant connecting with members at multiple touch points over the past year, and ensuring that vaccine education was not their first conversation with their health plan during the pandemic, Chief Medical Officer William Shrank, M.D., said. “I don’t think any of our members see this as our first outreach,” Shrank said.” But bear in mind health plans better late than never.
- The Society for Human Resource Management discusses how employers can take steps now to reduce pandemic fatigue in their employees.
From the general healthcare front —
- The Wall Street Journal informs us that “A federal medical panel is calling for a significant expansion of CT scanning for smokers to detect lung cancer, citing studies that found the imaging studies can save more lives than previously known. The U.S. Preventive Services Task Force is advising people ages 50 to 80 to get the screening if they have smoked on average a pack of cigarettes daily for 20 years, and who currently smoke or have quit within the past 15 years. The panel’s previous recommendation, in 2013, recommended people get screened between ages 55 and 80, and have smoked the equivalent of a pack of cigarettes a day for 30 years, and currently smoke or have quit within the past 15 years.” The USPSTF recommendation will result in FEHB coverage of the CT scan for FEHBP members in the expanded group without member cost sharing in 2023.
- Health Affairs helpfully reports “Bundled payment has shown promise in reducing medical spending while maintaining quality. However, its impact among commercially insured populations has not been well studied. We examined the impacts on episode cost and patient cost sharing of a program that applies bundled payments for orthopedic and surgical procedures in a commercially insured population. The program we studied negotiates preferred prices for selected providers that cover the procedure and all related care within a thirty-day period after the procedure and waives cost sharing for patients who receive care from these providers. After implementation, episode prices for three selected surgical procedures declined by $4,229, a 10.7 percent relative reduction. Employers captured approximately 85 percent of the savings, or $3,582 per episode (a 9.5 percent relative decrease), and patient cost-sharing payments decreased by $498 per episode (a 27.7 percent relative decrease).” Interesting.
- Health Payer Intelligence discusses how payers can take action against racial and ethnic healthcare disparities. Because “the payer industry’s core function is to pay for medical care, insurers can play a key role in overturning local care disparities through their payment strategies, offering funds to organizations that reduce disparities, and by spending locally in a way that is conscious of systemic inequities by purchasing from black and minority-owned businesses,” [Kedar] Mate [president and chief executive officer at the Institute for Healthcare Improvement (IHI), president of the IHI Lucian Leape Institute, and a member of the faculty at Weill Cornell Medical College] said.
From the healthcare industry front
- Forbes informs us that “In the largest advertising blitz ever undertaken by the health insurance lobby, America’s Health Insurance Plans will spend at least $10 million on a national education campaign to show how health plans are ‘are working together to deliver affordable and accessible care and coverage.’”
- Fierce Healthcare explains that “If there was one key word to come out of Cigna’s investor day on Monday [March 8], it would be “growth.” The insurer spotlighted its ambitions to expand across its enterprise, from its insurance plans to pharmacy to digital tools, at the virtual event for investors Monday morning. CEO David Cordani said that its growth plans “fuel our purpose.”
- Fierce Healthcare also reports that “COVID-19 accelerated a number of trends already brewing in the healthcare industry, and that’s not likely to change this year, according to a new report from CVS Health. The healthcare giant released its annual Health Trends Report on Tuesday [March 9], and the analysis projects several industry trends that are likely to define 2021 in healthcare, ranging from technology to behavioral health to affordability. “We are facing a challenging time, but also one of great hope and promise,” CVS CEO Karen Lynch said in the report. “As the pandemic eventually passes, its lessons will serve to make our health system more agile and more responsive to the needs of consumers.”
- Drug Channels lists that fifteen largest U.S. pharmacy chains in terms of market share and revenue. CVS Health leads the pack.
From the regulatory front, the Department of Health and Human Services’ Office for Civil Rights (“OCR”) announced today “a 45-day extension of the public comment period for the Notice of Proposed Rulemaking (NPRM) to modify the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. OCR first released the NPRM to the public on the HHS website on December 10, 2020, and it was published in the Federal Register on January 21, 2021. The 45-day extension moves the current deadline for the public to submit comments from March 22, 2021, to May 6, 2021. The notice of extension of the comment period is available at https://public-inspection.federalregister.gov/2021-05021.pdf – PDF.” The FEHBlog is pleased that the Biden Administration is giving serious consideration to this proposed rule.