Tuesday’s Tidbits

From Capitol Hill, Roll Call reports that
The House and Senate are moving swiftly toward passing legislation introduced Tuesday that would limit Senate debate on debt limit legislation to 10 hours, creating a loophole in that chamber’s 60-vote legislative filibuster rules.in his Morning’s column in the New York Times andor a vote Tuesday night, Speaker Nancy Pelosi wrote in a letter to lawmakers, along with a revised fiscal 2022 defense policy bill that would be sent to the Senate separately.
The two legislative vehicles are unrelated bills that previously passed both chambers with amendments; using them to carry the budget and defense measures allows Senate leaders to avoid a time-consuming motion to proceed in that chamber. Instead, only one cloture vote per bill would be needed.
Senate Minority Leader Mitch McConnell, who briefed his caucus at lunch on Tuesday, blessed the arrangement in comments to reporters. He said the new debt limit measure could pass as early as Thursday, after the Senate clears the bill to create an expedited process.
“I’m confident that this particular procedure coupled with the avoidance of Medicare cuts will achieve enough Republican support to clear the 60 vote threshold,” McConnell said.
If Congress accomplishes all of these actions, it may just call it quits at the end of this week which was the original schedule. A delay in Medicare cuts is extremely important to the medical facility and provider professional associations.
On the COVID vaccine mandate front, a federal district judge in Georgia today ordered a nationwide preliminary injunction against enforcement of the federal government contractor mandate per Govexec which adds
The Biden administration’s vaccine rule for private businesses and vaccine mandate for Medicare- and Medicaid-certified providers and suppliers are also temporarily blocked by courts. So far, the vaccine mandate for federal employees has not been stopped.
This PI applies to all FEHB plan contractors and subcontractors.
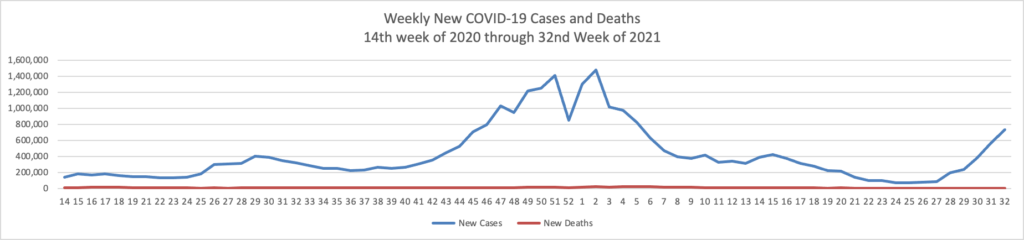
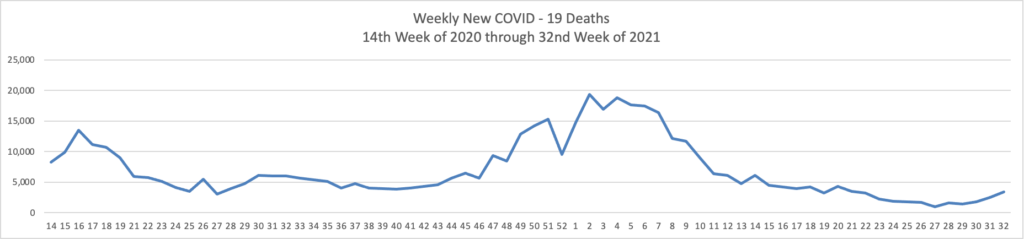
From the Delta variant front, David Leonhardt who is the FEHBlog’s go-to COVID columnists recommends in his Morning column in the New York Times today
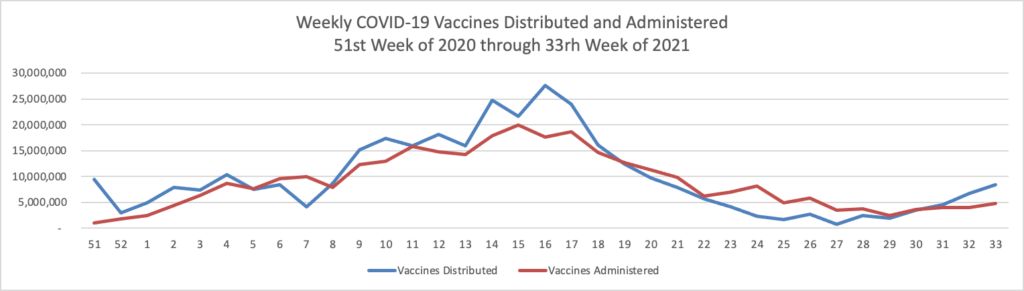
For now, vaccinated people can reasonably continue to behave as they were — but many should feel urgency about getting booster shots. Older people and others who are vulnerable, like people receiving cancer treatment, should continue to be careful and ask people around them to test frequently.
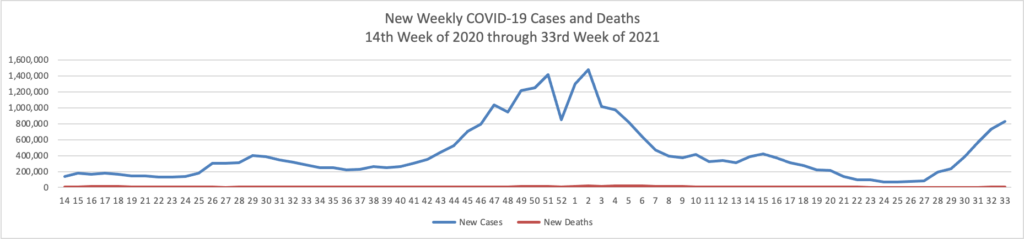
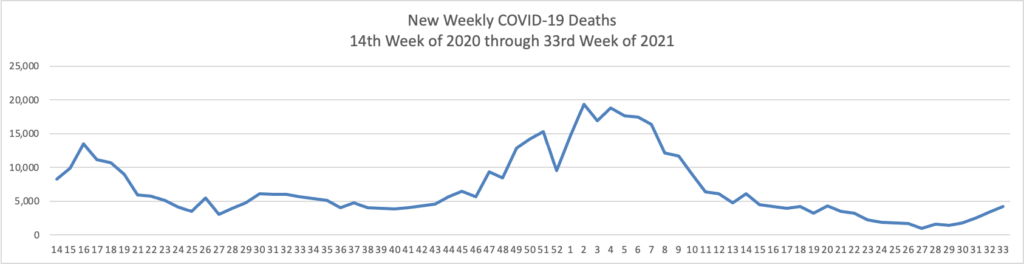
Unvaccinated people remain at substantial risk of serious illness. About 1,000 Americans have been dying each day of Covid in recent weeks, the vast majority of them unvaccinated.
From the Rx coverage front, Drug Channels released its
annual deep dive into employer-sponsored coverage for prescription drugs.
For 2021, employers backed away slightly from high-deductible health plans. However, their pharmacy benefit designs increased the use of coinsurance for specialty and fourth-tier drugs. These designs have significantly raised patients’ out-of-pocket obligations and are likely to have reduced adherence.
Manufacturers’ patient support funds help offset patients’ higher expenses. But employer plans are rapidly adopting copay accumulators, which allow payers and PBMs to absorb these funds.
From the health benefits trends front, the Society for Human Resource Management informs us that
Three-quarters of health insurers say that managing a health plan’s network of care providers is critical to controlling rising medical costs.
The finding is from consultancy Willis Towers Watson’s 2022 Global Medical Trends Survey, conducted from July through September 2021 among 209 leading insurers globally.
The plan features mostly likely to keep costs under control, insuers said, were:
— Contracting with high-quality, cost-competitive doctors and hospitals for in-network coverage (cited by 75 percent of respondents).
— Requiring preapproval for scheduled inpatient services (67 percent).
— Offering telehealth services (63 percent).
Telehealth or virtual care rose to the third spot from the fifth position last year, “a sign that more insurers see potential savings from remote options for diagnosing and treating patients,” according to the report.
Yesterday was the deadline for submitting public comments on the the second No Surprises Act interim final rule, which concerns the independent dispute resolution process. For a ying and yang take on the comments, here are links to American Hospital Association’s comments and to AHIP’s comments.
Let’s wrap it up with a bunch of HHS tidbits
- HHS today announced its plan to “propose a national “Birthing-Friendly” hospital designation on the Hospital Compare section of the CMS Care Compare website, and also encourages states to provide 12 months postpartum coverage to people with Medicaid and CHIP.”
- The National Institutes of Health reported that “Researchers identified brain cells that help suppress hunger and regulate food intake” and that “The findings may help lead to better treatments for excessive eating and obesity.”
- NIH also announced “The winners of the National Institutes of Health’s Decoding Maternal Morbidity Data Challenge were announced today in conjunction with the White House “day of action” on maternal health. Twelve prizes were awarded to seven winners who proposed innovative solutions to identify risk factors in first-time pregnancies. Without a prior pregnancy for comparison, it is difficult to identify risks for adverse pregnancy outcomes. Early detection of these risks can help reduce pregnancy complications and prevent maternal deaths.”
- The Agency for Healthcare Quality and Researched released
A final report on strategies to improve patient safety and reduce medical errors has been delivered to Congress by the U.S. Department of Health and Human Services in consultation with AHRQ. Required by the Patient Safety Act of 2005, the report was made available for public review and comment and review by the National Academy of Medicine. It outlined several strategies to accelerate progress in improving patient safety, including using analytic approaches in patient safety research, measurement, and practice improvement to monitor risk; implementing evidence-based practices into real-world settings through clinically useful tools and infrastructure; encouraging the development of learning health systems that integrate continuous learning and improvement in day-to-day operations; and encouraging the use of patient safety strategies outlined in the National Action Plan by the National Steering Committee for Patient Safety.
Access the final report, “Strategies to Improve Patient Safety: Final Report to Congress Required by the Patient Safety and Quality Improvement Act of 2005” (PDF, 1.16 MB).
- The Centers for Disease Control “announced today that it has awarded $22 million to nearly 30 organizations around the world to combat antimicrobial resistance (AR) and other healthcare threats through the establishment of two new networks—the Global Action in Healthcare Network (GAIHN) and the Global AR Laboratory and Response Network (Global AR Lab & Response Network).”
- Yahoo News reports that
Citing mounting evidence of ongoing harm, U.S. Surgeon General Vivek H. Murthy on Tuesday issued a public health advisory on the mental health challenges confronting youth, a rare warning and call to action to address what he called an emerging crisis exacerbated by pandemic hardships.
Symptoms of depression and anxiety have doubled during the pandemic, with 25% of youth experiencing depressive symptoms and 20% experiencing anxiety symptoms, according to Murthy’s 53-page advisory. There also appear to be increases in negative emotions or behaviors such as impulsivity and irritability — associated with conditions such as attention deficit hyperactivity disorder or ADHD.
And, in early 2021, emergency department visits in the United States for suspected suicide attempts were 51% higher for adolescent girls and 4% higher for adolescent boys compared to the same time period in early 2019, according to research cited in the advisory.