Tuesday Tidbits

From Washington, DC,
- Federal News Network reports on the Postal Service Health Benefits Program supplemental rule creating a Medicare Part D EGWP mandate for Postal annuitants over 65, other than those living abroad.
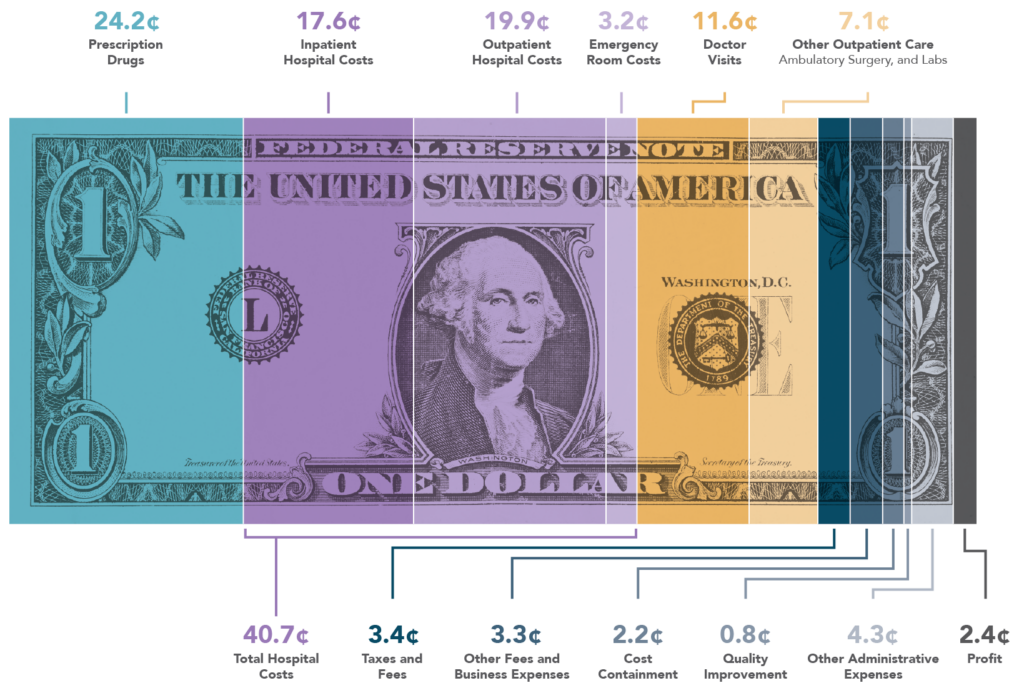
- While the FEHBlog thinks that the new, improved 2025 version of Medicare Part D is a good deal for FEHB and PSHB annuitants over age 65, even for those with the IRMAA tax or manufacturer coupons, the FEHBlog objects to the OPM mandate because it penalizes annuitants who opt out of the Plan’s Part D EGWP by barring them from the Plan’s prescription drug benefits without any premium reduction. Although FEHB plans do include penalties for failing to use hospital pre-certification, for example, those penalties top out at $500. Prescription drugs represent 24 cents out of every healthcare dollar according to AHIP. If Congress had intended that OPM impose such a hefty penalty, it would have said so in the Postal Reform Act. The law, however, is silent.
- FedSmith offers advice on the upcoming Open Season while FedWeek explains the pros and cons about FEHB / PSHP high deductible plans with health savings accounts.
- Per a CMS press release,
- “The Centers for Medicare & Medicaid Services (CMS) announced today that the Medicare Shared Savings Program (Shared Savings Program) continues to save Medicare money while supporting high-quality care. The Shared Savings Program yielded more than $2.1 billion in net savings in 2023 — the largest savings in the Shared Savings Program’s history. In addition, Shared Savings Program Accountable Care Organizations (ACOs) are providing higher-quality care and supporting policies CMS has adopted to enhance primary care, expand access to accountable care to underserved communities, and prioritize quality care for common chronic conditions.
- “In 2023, ACOs in the Shared Savings Program earned shared savings payments (also known as performance payments) totaling $3.1 billion, the highest since the program’s inception more than 10 years ago. In addition, ACOs scored better on many quality measures than other types of physician groups and continued to demonstrate quality improvement. ACOs led by primary care clinicians had significantly higher net per capita savings than ACOs with a smaller proportion of primary care clinicians. These results continue to underscore how important primary care is to the success of the Shared Savings Program.”
- Healthcare Dive tells us,
- “Oracle Health will apply to become a Qualified Health Information Network under the federal government’s health data exchange framework, the technology giant said Monday.
- “TEFCA, or the Trusted Exchange Framework and Common Agreement, uses QHINs — which can represent dozens or hundreds of health systems, public health agencies, payers and health IT vendors — to support health information sharing, according to the HHS’ Assistant Secretary for Technology Policy/Office of the National Coordinator for Health IT.
- “To get official designation, QHINs have to complete technology and security testing and agree to the data sharing rules before being onboarded. TEFCA went live in December with five QHINs, and two more organizations were approved early this year.”
From the public health and medical research front,
- Fierce Pharma informs us,
- “A small tweak in the dosing regimen of Eli Lilly’s Alzheimer’s disease drug Kisunla has reduced brain swelling of patients in a trial, the company said Tuesday.
- “In the phase 3 study, 14% of patients who were on the altered dosing plan experienced brain swelling (ARIA-E) events at Week 24 versus 24% of those who received the standard dosing of Kisunla, which was approved by the FDA in July.
- ‘The difference adds up to a 41% reduction in ARIA-E and could lead to a label change and help convince doctors to prescribe the anti-amyloid therapy, which is competing with another Alzheimer’s drug in its class, Eisai and Biogen’s Leqembi.”
- Per MedTech Dive,
- “Edwards Lifesciences’ Early TAVR trial results showed asymptomatic patients with severe aortic stenosis had better outcomes after transcatheter aortic valve replacement than under routine clinical surveillance.
- “Analysts said the positive data could help Edwards reaccelerate growth in its TAVR business, where sales have slowed in recent quarters. The data were presented Monday at the Cardiovascular Research Foundation’s Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium and published simultaneously in The New England Journal of Medicine.
- “The study is the first randomized, controlled trial to look at early intervention with TAVR as a strategy in patients with asymptomatic severe aortic stenosis, according to Edwards. The study was funded by Edwards.”
- Per a company press release,
- “Shionogi & Co., Ltd. (Head Office: Osaka, Japan; Chief Executive Officer: Isao Teshirogi, Ph.D.; hereafter “Shionogi”) today announced that its double-blind, randomized, placebo-controlled global Phase 3 study, Stopping COVID-19 pRogression with early Protease InhibitOr treatment – Post Exposure Prophylaxis (SCORPIO-PEP), met its primary endpoint. Once-daily ensitrelvir (Generic name: ensitrelvir fumaric acid, Code No.: S-217622, hereafter “ensitrelvir”) demonstrated a statistically significant reduction in the proportion of participants with symptomatic SARS-CoV-2 infection after exposure to household contacts with COVID-19 when compared to placebo. Specifically, the primary endpoint assessed COVID-19 symptoms onset through Day 10. Ensitrelvir was well tolerated by study participants and no new safety concerns were identified.
- “Ensitrelvir is an investigational oral antiviral that suppresses the replication of SARS-CoV-2 by selectively inhibiting the viral 3CL protease. Ensitrelvir was granted Fast Track designation by the U.S. Food and Drug Administration in 2023 for the treatment of COVID-19. In Japan, ensitrelvir, known as Xocova®, received emergency regulatory approval in 2022 and full approval in March 2024 for the treatment of COVID-19. Ensitrelvir was also made available in Singapore based on the Special Access Route application in 2023. It remains an investigational drug outside of Japan and Singapore.
- “COVID-19 remains an important public health priority, yet there are currently no oral antiviral medications approved for post-exposure prophylactic use. There is a need for convenient, preventive approaches to protect ourselves and those close to us from contracting SARS-CoV-2,” said Simon Portsmouth, MD, FRCP, Senior Vice President, Head of Clinical Development. “These data demonstrate a new potential for post exposure prophylactic use of ensitrelvir, expanding on the breadth of clinical and real-world evidence that establish its activity in those infected with SARS-CoV-2.”
- AHRQ’s Medical Expenditures Panel Survey lets us know,
- Among adults who reported ever having COVID-19, 13.7 percent reported ever having long COVID.
- Women were more likely than men to report ever having long COVID (16.5% vs. 10.5%).
- Adults aged 18-34 were less likely than all other age groups to report ever having long COVID (9.8% vs. 13.5%-17.9%).
- Adults living in high-income households were less likely to report ever having long COVID (11.0%) than those living in middle-income households (15.6%), low-income or near poor households (17.4%), and those living in poor households (17.2%).
- Adults living in a metropolitan statistical area reported lower rates of ever having long COVID than those living outside of a metropolitan statistical area (12.7% vs. 19.7%).
- MedPage Today points out,
- “Elevated body mass index (BMI) in children and young adults was associated with an increased risk of post-acute sequelae of SARS-CoV-2 infection (PASC), or long COVID, a large retrospective cohort study suggested.
- “Those with obesity had a 25.4% increased risk of long COVID (relative risk [RR] 1.25, 95% CI 1.06-1.48) and those with severe obesity had a 42.1% increased risk (RR 1.42, 95% CI 1.25-1.61) compared with children and young adults who had healthy weight, reported Yong Chen, PhD, of the University of Pennsylvania in Philadelphia, and colleagues.
- “Similarly, there was an increased likelihood of encountering any manifestation of potential long COVID symptoms and conditions among those with obesity (RR 1.11, 95% CI 1.06-1.15) and severe obesity (RR 1.17, 95% CI 1.14-1.21), they said in JAMA Network Openopens in a new tab or window.
- “To our knowledge, this retrospective cohort study is the first and the largest to explore the association of BMI status with PASC among the pediatric population,” Chen and co-authors wrote. “The findings suggest that PASC may lead to poorer long-term quality of life, affecting physical health, educational achievement, and social development; this underscores the importance of early identification, prevention, and targeted interventions to mitigate these risks.”
- The U.S. Preventive Services Task Force (USPSTF) has opened for a public comment the following recommendations:
- Population: Pregnant or postpartum persons and women of reproductive age
- Recommendation: The USPSTF recommends that clinicians screen for intimate partner violence (IPV) in pregnant and postpartum persons and women of reproductive age. See the “Practice Considerations” section for information on evidence-based multicomponent interventions and for information on IPV in men.
- Grade: B
Population: Older or vulnerable adults
Recommendation The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for caregiver abuse and neglect in older or vulnerable adults. See the “Practice Considerations” section for additional information.
Grade: I (inconclusive) - “In 2018, the USPSTF recommended that clinicians screen for IPV in women of reproductive age and provide or refer women who screen positive to ongoing support services. The USPSTF also concluded that the evidence was insufficient to assess the balance of benefits and harms of screening for abuse and neglect in all older or vulnerable adults. The current draft recommendation statement is consistent with the 2018 recommendation. To highlight that the evidence base focused on pregnant and postpartum persons, the USPSTF emphasized this population in this draft recommendation statement. For abuse of older or vulnerable adults, the term “caregiver” was added before abuse or neglect when appropriate to clarify when the focus was on screening for abuse or neglect perpetrated by a caregiver or someone they trust.”
- The public comment deadline is November 25, 2024.
- Healio relates that “A modified screening with additional questions about suicidal ideation was better at predicting suicide attempts among adolescents than the standard questionnaire, according to findings published in JAMA Network Open.“
- The Wall Street Journal notes,
- “In Appalachia, in the heart of one of the earliest and deadliest waves of the opioid crisis, doctors at West Virginia University’s Rockefeller Neuroscience Institute are conducting a radical experiment. Using focused ultrasound waves, they are resetting cells inside the brain’s reward center, the nucleus accumbens. They hope the procedure can treat addictions ranging from drugs like opioids and methamphetamine to gambling and eating.
- “While neuroscientists have long defined addiction as a brain disease, tools to fight the U.S. drug crisis that is behind 100,000 overdose deaths a year have changed little in decades. Most treatment involves medications like methadone and buprenorphine to replace other opioids, or naltrexone to block the part of the brain that feels pleasure from alcohol or opioids. For many addictions, counseling and abstinence-based 12-step programs remain the go-to treatment.
- “At RNI’s 30-patient residential-treatment program, more than two-thirds of patients relapse within the first few weeks. Many illicit drugs, including meth and cannabis, don’t have any prescription medications to treat the addiction.
- “Now, the institute’s trial using ultrasound is a peek at a future that treats the physical brain, rather than using medication or behavioral approaches to alter outcomes. “We need to inject technology into this,” said Dr. Ali Rezai, a neurosurgeon and executive chair at the institute.
- “The RNI team is also studying a pill that monitors vital signs and releases overdose-reversal medication automatically in people who overdose. In another trial, they are monitoring the heart rates, emotions, sleep and cravings of thousands of drug users who are helping to train artificial intelligence to predict a relapse before it occurs, so that recovery coaches can intervene.”
From the U.S. healthcare business front,
- The Wall Street Journal reports,
- “Pfizer PFE punched back against activist investor Starboard Value on Tuesday, delivering positive quarterly results.
- “The pharmaceutical company raised its revenue outlook for the year to between $61 billion to $64 billion, up from $59.5 billion to $62.5 billion previously. It also raised its guidance on adjusted annual earnings per share to a range of $2.75 to $2.95, up from $2.45 to $2.65.
- “The encouraging third quarter comes as Pfizer faces pressure from activist investor Starboard, which says poor investments in research and dealmaking have helped destroy billions of dollars in market capitalization. The latest earnings highlight Pfizer’s third consecutive quarter with positive results—a bright spot that could bolster the drugmaker, and Chief Executive Albert Bourla’s efforts to revamp the company.
- ‘Pfizer also beat Wall Street’s expectations on quarterly sales and earnings. The company reported sales totaling $17.7 billion, driven by Covid-19 products and cancer medicines, up from the $14.9 billion forecast by analysts surveyed by FactSet. The Covid-19 antiviral Paxlovid generated $2.7 billion in quarterly sales, while its Covid-19 vaccine Comirnaty sold $1.4 billion, both topping analyst forecasts.”
- Modern Healthcare informs us,
- “CVS Health’s MinuteClinic is becoming an in-network primary care provider for select Aetna plan members.
- “Aetna commercial, individual and family health plan members in San Antonio, Houston, Atlanta and south Florida have the option to use MinuteClinic as an in-network primary care provider, with members in North Carolina becoming eligible in the coming weeks, said Dr. Creagh Milford, retail health president at CVS Health.
- “CVS has been investing in staffing, technology and training at its MinuteClinic sites for months to expand primary care services in certain markets chosen based on patient density, demographics and existing services in those areas, Milford said.
- “We’re seeing a lot of growth in the model,” Milford said. “Our ambition is to move the patient perception and the payer perception from one of an episodic, acute care model toward a longitudal, relationship-based primary care model.”
- “CVS is in talks with other health plans to grow the MinuteClinic primary care approach, he said.”
- STAT News tells us,
- “More than a year has passed since Dana-Farber Cancer Institute dumped Mass General Brigham for a rival hospital chain, but the state’s biggest health care system is making a push now to say when it comes to cancer care, MGB’s still got it.
- “Beginning in 2028, Dana-Farber will end its long and nationally acclaimed adult oncology partnership with Brigham and Women’s Hospital. Instead, it will team up with Beth Israel Deaconess Medical Center to open a new freestanding 300-bed, $1.68 billion cancer hospital in the Longwood Medical Area.
- “Dana-Farber’s announcement of the divorce in September 2023 stunned executives at the Brigham and rocked the hyper-competitive hospital industry. But now MGB is fighting back by creating what it calls the Mass General Brigham Cancer Institute, which the health system is trumpeting in an intensive marketing campaign.
- “The institute won’t be a freestanding hospital. But it will, for the first time, combine the expertise and resources of MGB’s two flagship hospitals, Massachusetts General Hospital and the Brigham, whose cancer operations were previously separated by a firewall because of the latter’s partnership with Dana-Farber.
- “What really started as a disruptive event a year ago, saying that Dana-Farber will be exiting after a few years, has now become a new opportunity for us to rethink how we deliver care,” O’Neil Britton, chief integration officer for MGB, said Monday at a round-table discussion at Massachusetts General Hospital with reporters.”
- Per Fierce Pharma,
- “Exactly three years after an initial FDA green light for the third-line treatment of leukemia, Novartis’ Scemblix has won an accelerated approval to treat newly diagnosed patients.
- “Tuesday, the FDA cleared Scemblix to treat patients with newly diagnosed Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in the chronic phase. The first-line nod marks an important step for the Gleevec follow-up on its way toward reaching the company’s peak sales projection of $3 billion.
- “Only about 15% of Ph+ CML patients reach the third-line treatment setting, Victor Bulto, Novartis’ U.S. president, noted in an interview with Fierce Pharma.
- “The Swiss drugmaker has its work cut out in this use. While Scemblix has quickly become the standard of care in third-line Ph+ CML because of a lack of alternative treatments, the first-line market will feature a couple hurdles for the new entrant.”
- Per BioPharma Dive,
- “Paragon Therapeutics, a biotechnology company creator with a web of spinouts, is taking a new startup public to develop an emerging type of cancer immunotherapy.
- “The startup, Crescent Biopharma, on Tuesday announced a reverse merger with GlycoMimetics, a struggling, publicly traded developer of oncology and inflammatory disease drugs. In support of the deal, the combined company has raised $200 million in financing from 17 major investment firms — among them Fairmount and Venrock Healthcare Capital Partners — and expects that money to keep it operating through 2027.
- “The new company will take the Crescent name, be about 97% owned by Crescent stockholders, and be led by the startup’s interim CEO and Fairmount venture partner Jonathan Violin. Its chief goal will be to advance a group of cancer medicines led by a dual-pronged immunotherapy that simultaneously targets the proteins PD-1 and VEGF.
- “Study results in September showed that approach could improve upon standard immunotherapy treatments, like Merck & Co.’s Keytruda. Drugs targeting PD-1 and VEGF have since drawn the interest of an array of biotech companies, of which Crescent is the latest to emerge.”
- and
- “GSK will pay $300 million to acquire a bispecific antibody from Shanghai-based Chimagen Biosciences that it believes has the potential to treat autoimmune diseases like lupus.
- “The drug, which is currently in Phase 1 testing for cancer in the U.S. and China, is what’s known as a “T cell engager.” It binds to two cell surface proteins called CD19 and CD20, which GSK notes could help deplete malfunctioning B cells.
- “In a Tuesday statement, GSK said it plans to begin a Phase 1 trial of Chimagen’s drug sometime next year, assuming the proposed licensing deal clears customary regulatory review.”