Weekend update
This coming week, the House of Representatives resumes Committee business and the Senate resumes both Committee business and floor voting. The Wall Street Journal adds that “After a two-week recess, senators return to Washington this week to determine the fate of much of President Biden’s roughly $4 trillion agenda. Senate Majority Leader Chuck Schumer (D., N.Y.) told Senate Democrats in a letter on Friday that he expects that the chamber will take up both a roughly $1 trillion infrastructure agreement and a resolution setting the parameters of a bill encompassing other Democratic priorities in the coming weeks.”
Returning to the President’s July 9, 2021, executive order on competition the accompanying Fact Sheet explains that with regard to healthcare:
BEGIN QUOTE
In the Order, the President:
- Directs the Food and Drug Administration to work with states and tribes to safely import prescription drugs from Canada, pursuant to the Medicare Modernization Act of 2003.
- Directs the Health and Human Services Administration (HHS) to increase support for generic and biosimilar drugs, which provide low-cost options for patients.
- Directs HHS to issue a comprehensive plan within 45 days to combat high prescription drug prices and price gouging.
- Encourages the FTC to ban “pay for delay” and similar agreements by rule.
Hearing Aids: Hearing aids are so expensive that only 14% of the approximately 48 million Americans with hearing loss use them. On average, they cost more than $5,000 per pair, and those costs are often not covered by health insurance. A major driver of the expense is that consumers must get them from a doctor or a specialist, even though experts agree that medical evaluation is not necessary. Rather, this requirement serves only as red tape and a barrier to more companies selling hearing aids. The four largest hearing aid manufacturers now control 84% of the market.
In 2017, Congress passed a bipartisan proposal to allow hearing aids to be sold over the counter. However, the Trump Administration Food and Drug Administration failed to issue the necessary rules that would actually allow hearing aids to be sold over the counter, leaving millions of Americans without low-cost options.
In the Order, the President:
- Directs HHS to consider issuing proposed rules within 120 days for allowing hearing aids to be sold over the counter.
Hospitals: Hospital consolidation has left many areas, especially rural communities, without good options for convenient and affordable healthcare service. Thanks to unchecked mergers, the ten largest healthcare systems now control a quarter of the market. Since 2010, 139 rural hospitals have shuttered, including a high of 19 last year, in the middle of a healthcare crisis. Research shows that hospitals in consolidated markets charge far higher prices than hospitals in markets with several competitors.
In the Order, the President:
- Underscores that hospital mergers can be harmful to patients and encourages the Justice Department and FTC to review and revise their merger guidelines to ensure patients are not harmed by such mergers.
- Directs HHS to support existing hospital price transparency rules and to finish implementing bipartisan federal legislation to address surprise hospital billing.
Health Insurance: Consolidation in the health insurance industry has meant that many consumers have little choice when it comes to selecting insurers. And even when there is some choice, comparison shopping is hard because plans offered on the exchanges are complicated—with different services covered or different deductibles.
In the Order, the President:
- Directs HHS to standardize plan options in the National Health Insurance Marketplace so people can comparison shop more easily.
END QUOTE
Roll Call and Healthcare Dive relate industry reaction to the order. The FEHBlog is not happy with drug importation from Canada directive because our population exceeds Canada’s by ten times. It’s a gimmic. Also the FEHBlog disagrees with standardizing plan designs which by definition inhibits competition. Also the objections to hospital and health insurer consolidation overlooks the fact that the Affordable Care Act largely has driven the consolidation, in the FEHBlog’s opinion.
The Health Affairs Blog discusses Centers for Medicare Services efforts to keep its Medicare Part B schedule current. The article explains
Currently, physician services in the US are priced by Medicare every January 1 in relative value units (RVUs). Every physician service is assigned a Medicare-allowed price in RVUs based on its work “intensity” defined by time, effort, skill, and stress relative to all other services. RVUs are converted to dollars via the Medicare “conversion factor,” which CMS sets annually. Total Medicare allowed payment for each service also includes RVUs for practice expenses and malpractice risk, which are theoretically unrelated to physician compensation.
Commercial insurers generally use the same RVU fee schedule as the basis for physician payments. Value-based payment models use Medicare valuations for calculating costs and payments.
In recent decades, technological advances have substantially expanded the number of procedural services, which are generally priced far above evaluation and management (E/M) services. As procedures are increasingly completed safely in less time, the RVU generation potential of procedurally oriented physician work has also grown. In contrast, the analogous expansion of therapeutic choices and medications that are at the core of E/M services have not been reflected by increased valuations. This has contributed to widened income gaps between proceduralists and non-proceduralists, leading to the lack of incentives for trainees to enter lower-reimbursed specialties, including primary care, endocrinology, oncology, rheumatology, and infectious diseases.
Federal News Network reports that
After a slower January and February, federal retirement seems to be picking back up in the first half of 2021 compared to 2020. June stats from the Office of Personnel Management for newly filed claims showed last month was higher than a year ago, when the pandemic was in full effect.
OPM received 7,264 new retirement claims last month compared to 6,555 new claims in June 2020 — a 10.8% increase. March, April and May each saw year-over-year increases ranging from 15.6%- 47.2%.
Processing them all is a different matter, as last month saw 6,884 claims processed compared to 7,300 processed in June 2020 — a 5.7% decrease. After peaking in March, the claims backlog has been moving downward, but at 24,999 last month is still 30.3% higher than a year ago and 92.3% higher than the steady state goal of 13,000 claims — nearly double.
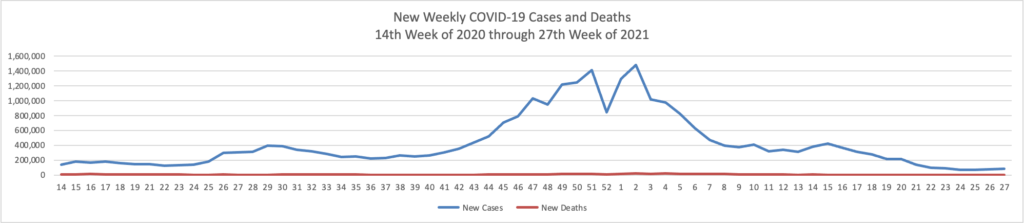
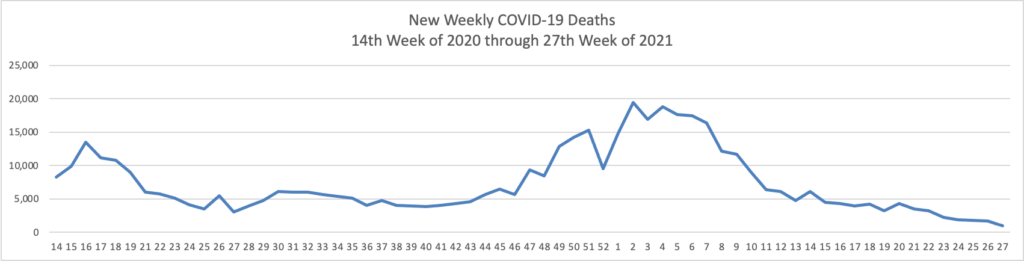
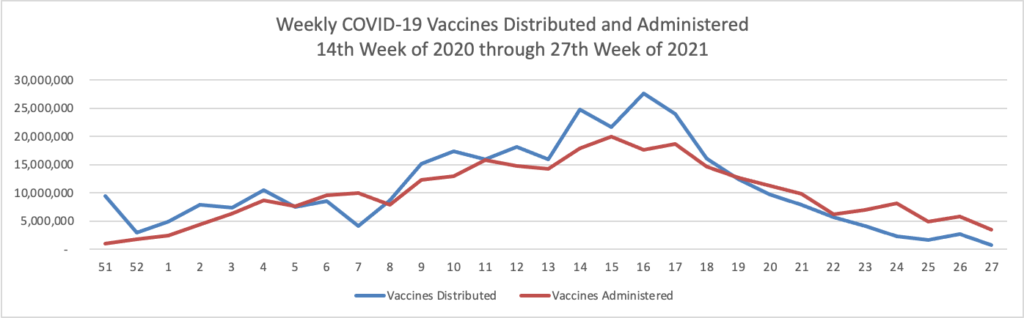
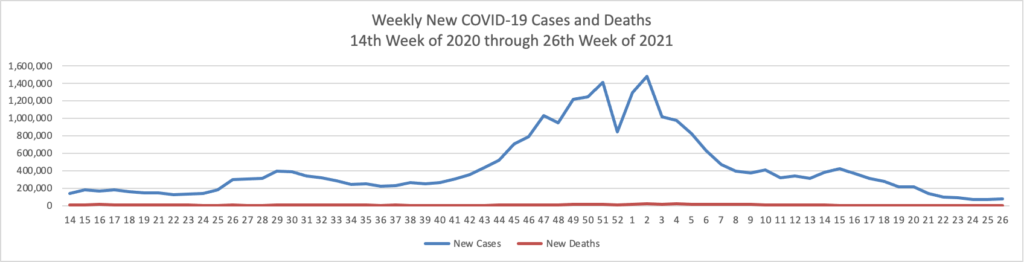
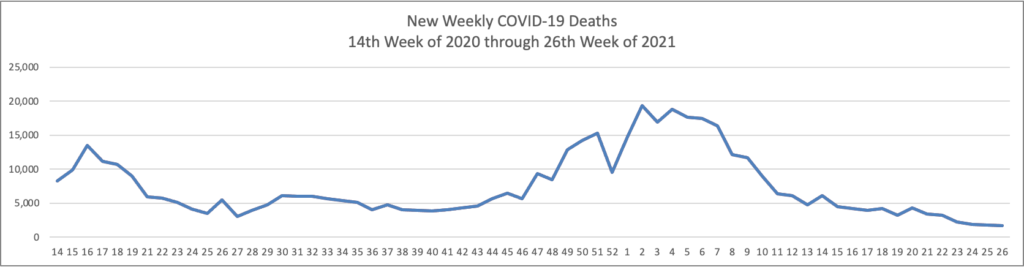
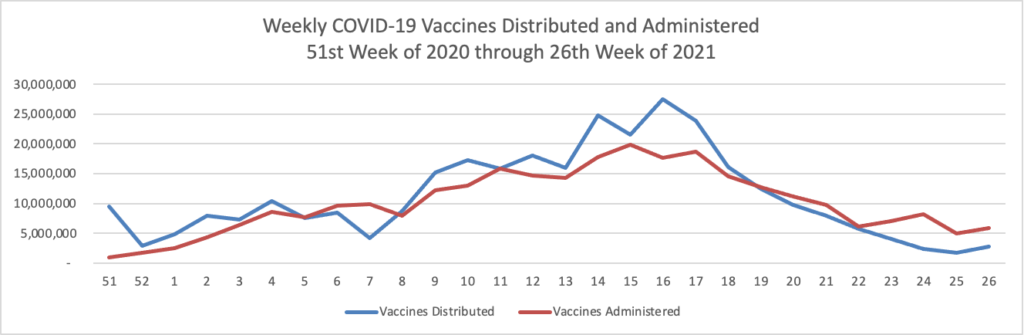
From the COVID-19 front, Bloomberg informs us that “In the U.S., 334 million doses have been given so far. In the last week, an average of 506,771 doses per day were administered.” According to the CDC, 159.3 million Americans are fully vaccinated. The Wall Street Journal adds that “[While] millions of Americans have rolled up their sleeves to get vaccinated against Covid-19, one group is well behind: young adults.
Their reluctance is a significant part of why the U.S. missed the Biden administration’s goal of getting 70% of the adult population a first dose by July 4, and it is impeding efforts to develop the communitywide immunity sought to move past the pandemic and fend off Delta and other variants.
Now government health authorities are dialing up efforts encouraging 18- to 29-year-olds to get vaccinated.
Turning to the telehealth front,
- The American Medical Association provides tips on how physicians can being warmth to the virtual visit.
- Healio informs us that “New research suggests a letter may be all that it takes to lower the number of telehealth no-shows among older patients, even during a pandemic. * * * ‘The letter was a very simple reminder, stating ‘You have an upcoming telehealth visit with your doctor’ and included the date and a range of time that the provider would call, typically a 30-minute period,’ [researcher / physician Sarah] King said in the interview. * * * Overall, the researchers said their intervention was associated with a 33.1% drop in the telehealth no-show rate and an 8% drop in the no-show rate for in-person visits.”