Tuesday Tidbits

From the Delta variant front —
Medscape reports on yesterday’s CDC Advisory Committee on Immunization Practices meeting:
“Vaccines remain effective in preventing hospitalization and severe disease but might be less effective in preventing infection or milder symptomatic illness,” Sara Oliver, MD, the CDC scientist who presented the information [about the mRNA vaccines], told the committee.
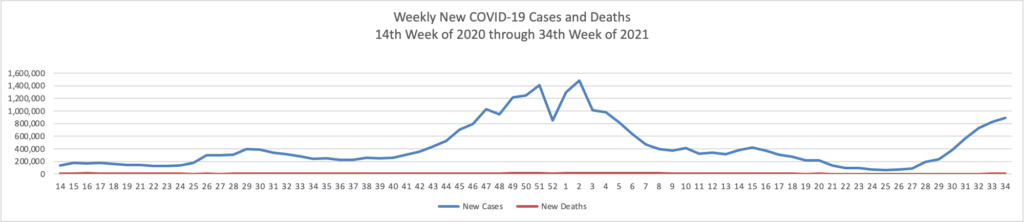
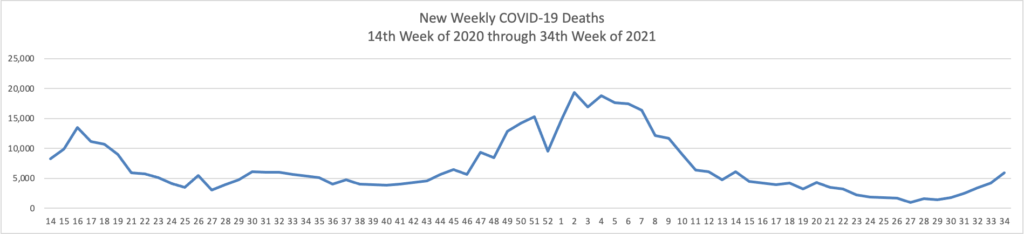
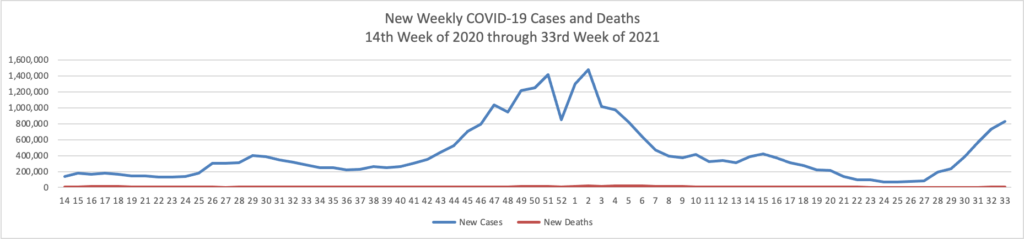
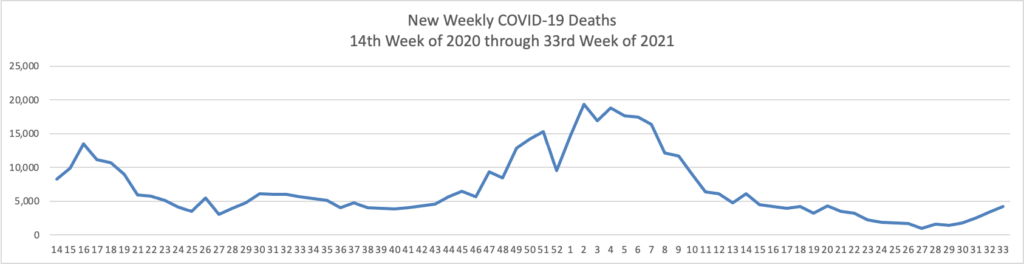
In a new data analysis released by the CDC on Sunday, unvaccinated adults were 17 times more likely to be hospitalized than vaccinated adults. Hospitalization rates were higher for unvaccinated people in all age groups.
Among the fully vaccinated, people who were hospitalized were much older, more likely to be nursing home residents and more likely to have three or more underlying medical conditions. Nearly a third had immunosuppressive conditions.
Healthcare Dive tells us that
The U.S. government will resume distribution of Eli Lilly’s COVID-19 antibody drug combination in a number of states, HHS said Friday, as cases and hospitalizations in the U.S. are driven higher by the spread of the delta variant.The decision comes roughly two months after administration of Lilly’s therapy was halted by U.S. officials due to concerns of reduced efficacy against coronavirus infections stemming from the beta and gamma variants. Those are now much less prevalent compared with delta, which laboratory testing has shown remains susceptible to treatment with the dual-antibody therapy, HHS said.
Fierce Pharma reports that
Last week’s FDA approval of Pfizer’s Comirnaty vaccine boosted consumer confidence among both vaccinated and unvaccinated people.
A Harris Poll survey over the weekend found that 80% of Americans who were aware of the approval now have more confidence in it. Even more encouraging? Almost half (49%) of unvaccinated people who heard about the approval said they will “probably” or “definitely” get vaccinated.
Overall awareness of the Pfizer approval was high—79% of those surveyed by The Harris Poll were aware of the FDA thumbs-up.
From the telehealth front, Kaiser Health News discusses re-emerging state law barriers to telehealth. “’The whole challenge is to ensure maximum access to health while assuring quality,’said Barak Richman, a Duke University law professor, who said laws and policies haven’t been updated to reflect new technological realities partly because state boards want to hang onto their authority.” The article provides an overview of options available to doctors and state boards.
From the miscellany front
- Beckers Hospital Review unveils six big ideas in health innovation. For example, Jason Joseph. Senior Vice President and Chief Digital and Information Officer at Spectrum Health (Grand Rapids, Mich.). As we innovate, we are forcing hidden barriers into the light via experimentation. We saw so many of these barriers uncovered within health care, such as lack of connectivity, digital competency, and the need for comprehensive managed workflow. We have shined a spotlight on how much of healthcare relies on people and inconsistent manual processes to get through the system. That needs to change, and that also requires changing a leader’s traditional mindset.
- The Agency for Healthcare Quality and Research is offering a toolkit to help healthcare providers and possible health plans get patients and members engaged with the diagnosis process.
The toolkit contains two strategies, Be The Expert On You and 60 Seconds To Improve Diagnostic Safety. When paired together, these strategies enhance communication and information sharing within the patient-provider encounter to improve diagnostic safety. Each strategy contains practical materials to support adoption of the strategy within office-based practices.
Be The Expert On You is a patient-facing strategy that prepares patients and their families to tell their personal health stories in a clear, concise way. Research suggests that 79 percent of diagnostic errors are related to the patient-clinician encounter and up to 56 percent of these errors are related to miscommunication during the encounter. Environmental scan findings show that inviting patients to share their entire health story, uninterrupted, and in a way that gives clinicians the information they need can reduce diagnostic errors.
60 Seconds To Improve Diagnostic Safety prepares providers to practice deep and reflective listening for one minute at the start of a patient-encounter. Research suggests that patients are interrupted by their providers in the first 11 to 18 seconds of telling their diagnostic story. Diagnostic safety can be improved when a provider allows a patient to tell his or her health story without interruption for one minute, and then asks questions to deepen understanding.
- Not every innovative idea makes sense to implement though. The President and Democrat leadership in Congress want the Centers for Medicare Services to “negotiate” drug prices for Medicare Part D plans. Regulatory Focus informs us that “A new drug development model released by the Congressional Budget Office (CBO) estimates a Medicare drug pricing bill like the one proposed by Democrats in the US House of Representatives could result in between 21 and 59 fewer drugs brought to market over the next three decades.”