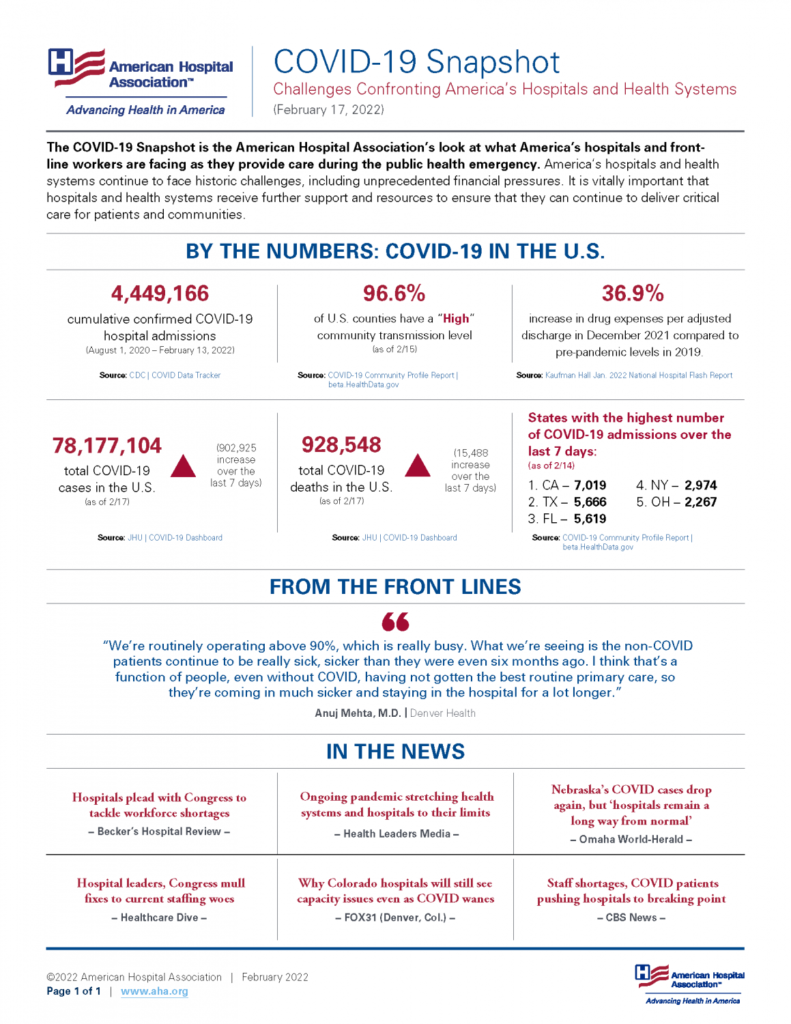
Friday Stats and More
Based on the CDC’s Covid Data Tracker and using Thursday as the first day of the week, here are the FEHBlog’s weekly charts of new Covid cases and deaths beginning with the 27th week of 2021 and ending with the 7th week of 2022.


At last, both charts are headed down.
Here’s the FEHBlog’s weekly chart of Covid vaccinations distributed and administered beginning with the 51st week of 2020, when the vaccination program launched, and the 7th week of this year.

As of today, nearly 75% of Americans aged 18 and older (74.7%) are fully vaccinated, and 46.5% of Americans in that age group are boostered.
Precision Vaccines reports
More than one year after the first COVID-19 vaccine was administered in the United States, new data from a recent survey conducted online by The Harris Poll show 73% of consumers would like to see additional COVID-19 vaccines become available.
Currently, the World Health Organization has listed ten different COVID-19 vaccines based on various technologies.
But the U.S. FDA has only authorized/approved three COVID-19 vaccines.
Moreover, consumers would like to see COVID-19 vaccines developed from traditional methods, such as technologies used to produce diphtheria, mumps, chickenpox, or polio vaccines.
Novavax’s traditionally developed Covid vaccine is pending Food and Drug Administration emergency use authorization. The FDA’s Vaccines Advisory Committee will consider the EUA application first. That Committee’s next meeting will be held on March 3, 2022. The agenda is not available yet.
In great news, Bloomberg reports
[W]ith [Covid] cases plummeting thanks to the omicron decline, and government orders continuing to roll into pharmacies, doctors and health officials in New York and across the country say the supply is looking plentiful in many areas.
“You have lots of the drug and very few cases — that’s the best place you could possibly be,” says Infectious Diseases Society of America spokesman Aaron E. Glatt. The pills are broadly available, he said, though not at every corner drugstore.
The federal government tracks Paxlovid on a public-facing website that names which pharmacies have it, and a federal website for health-care providers indicates more than 130,000 courses are available.
Dare we hope that the country will be prepared for the next Covid variant if it crops up?
Here are links to the CDC’s Covid Data Tracker weekly review and its FluView. The top line of FluView reads, “Sporadic influenza activity continues across the country. In some areas, influenza activity is increasing.” The current winter continues to mirror the 2020-21 winter, with Omicron edging out the flu.
From the Covid fallout front, Health Payer Intelligence informs us
Senior members might continue to see canceled or delayed care even as coronavirus cases decline, a trend which has had significant impacts on payer revenues and spending in 2020 and 2021, a report from the University of Michigan’s National Poll on Healthy Aging found.
Nearly three out of ten adults over 50 (28 percent) delayed care due to the coronavirus pandemic in 2021.
“Whether they chose to postpone or their provider did, these patients missed opportunities for preventive care and for early detection and effective management of chronic conditions, not to mention operations and procedures to address a pressing health need,” said Jeffrey Kullgren, MD, associate director of the poll and a health care researcher and associate professor of internal medicine at Michigan Medicine in the University of Michigan’s academic medical center.
The National Poll on Healthy Aging surveyed 1,011 adults ages 50 and older in late January 2022. Seniors could complete the survey by phone or online.
Considering the FEHB’s demographics, this report should be relevant to FEHB carriers.
Speaking earlier of the FDA, here is a link to the FDA’s roundup for February 18, 2022. Moreover, Fierce Healthcare reports
Hospitals on average charge double the price for the same drugs compared to those offered by specialty pharmacies, according to a new insurer-funded study released as federal regulators ponder a probe into the pharmacy benefit management industry.
The study (PDF), released Wednesday by insurance lobbying group AHIP, comes as specialty pharmacies have grown in use among PBMs and payers to dispense specialty products. The study was released a day before a scheduled meeting Thursday of the Federal Trade Commission on whether to probe the competitive impact of PBM contracts and how they could disadvantage independent and specialty pharmacies.
“The data are clear, specialty pharmacies lower patient costs by preventing hospitals and physicians from charging patients, families, and employers excessively high prices to buy and store specialty medicines themselves,” said Matt Eyles, president and CEO of AHIP, in a statement.
From the telehealth front, mHealth Intelligence notes
Though patients have previously made their preference for virtual waiting rooms over traditional ones known, new survey results show that a majority of telehealth patients would rather just be notified by a text or call when their doctor is ready to see them.
Doximity, an online networking service and telehealth platform for healthcare professionals, conducted the new survey last November, polling 2,000 US adults, of whom 1,000 identified as having a chronic illness.
As telehealth becomes integrated into care delivery, questions around patient preferences arise. The survey helps shed some light, showing that 79 percent of patients would prefer a call or text letting them know that their doctor is ready to see them versus having to wait in a virtual waiting room. Even among chronic illness patients only, an overwhelming majority (81 percent) would prefer to receive a call or text.
Patients also displayed a strong preference for familiarity with a provider. Overall, 83 percent of patients surveyed said they would wait one to three days to see their current doctor rather than seeing a new physician immediately.
If these statistics float your boat, you will find many more exciting telehealth stats in the article.