Tuesday’s Tidbits

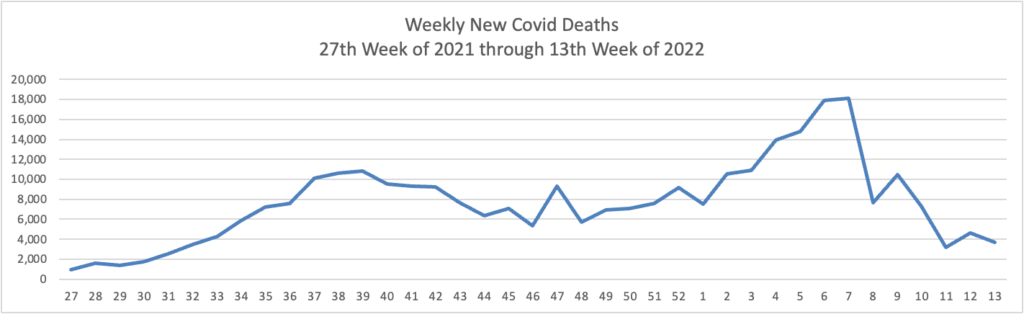
From the Omicron and siblings front
Beckers Hospital Review informs us
The World Health Organization on April 11 said it is monitoring two new “sister variants” of the original omicron strain dubbed BA.4 and BA.5, according to global news network WION.
Whither BA.3?
A lot of news sources are offering reports on Stealth Omicron. We learn from the AP that Stealth Omicron is another sibling of BA.2.
It was given the “stealth” nickname because it looks like the earlier delta variant on certain PCR tests, says Kristen Coleman at the University of Maryland School of Public Health. The original omicron, by contrast, is easy to differentiate from delta because of a genetic quirk.
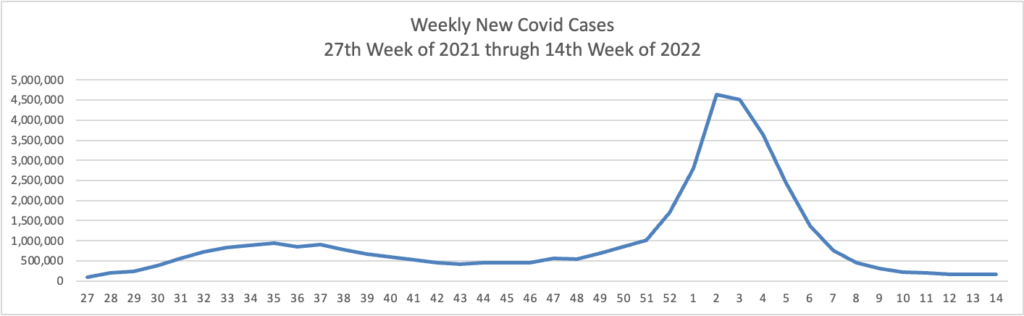
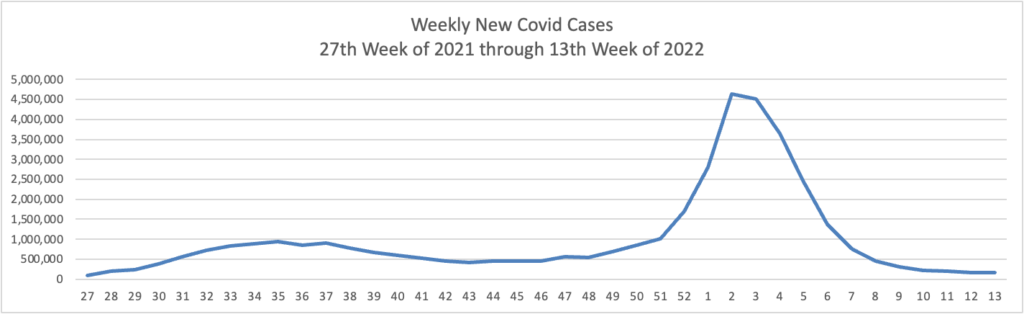
BA.2 is “now the dominant coronavirus version in the U.S. and more than five dozen other countries.”
From the Health and Human Services Department, we find an account of “Secretary Becerra and HHS Leaders Celebrating Black Maternal Health Week 2022.”
From the Centers for Disease Control department
- The CDC explains how diabetics can keep eating the cultural foods to which they are accustomed by taking a few preparation twists.
- The CDC reports
- Neisseria gonorrhoeae (gonorrhea), a common sexually transmitted infection (STI) that can result in life-threatening ectopic pregnancy and infertility, leads to more than an estimated half a million drug-resistant infections in the United States each year.
- With health departments in two states, CDC is expanding drug resistant-gonorrhea surveillance beyond traditional STI clinics and into emergency departments, where more people are seeking STI care.
- From 2018 through 2019, nearly one-third (29%) of patients with positive tests from the North Carolina site were diagnosed at emergency departments, and drug resistance testing uncovered eight cases of gonorrhea less likely to be successfully treated by one of two drugs in the recommended first line treatment at that time.
- Bloomberg adds “After sexually transmitted diseases fell during the early months of pandemic lockdowns and social distancing, the U.S. saw a resurgence of some of the most common infections through the end of 2020, according to a report.”
From the U.S. Preventive Services Task Force front, the Wall Street Journal tells us
All children should be screened for anxiety starting as young as 8 years old, government-backed experts recommended, providing fresh guidance as doctors and parents warn of a worsening mental-health crisis among young people in the pandemic’s wake.
The draft guidance marks the first time the U.S. Preventive Services Task Force has made a recommendation on screening children and adolescents for anxiety. The task force, a panel of independent, volunteer experts that makes recommendations on matters such as screening for diabetes and cancer, also reiterated on Tuesday its 2016 guidance that children between ages 12 and 18 years old should be screened for major depressive disorder.
“What the pandemic has done is, it exacerbated a pre-existing issue,” said Nasuh Malas, director of pediatric consultation and liaison psychiatry services at C.S. Mott Children’s Hospital in Ann Arbor, Mich., who isn’t on the task force. “These guidelines are a preliminary step to many, many steps that we need to take nationally as a community of people who are concerned about our youth.”
STAT News adds
After staying flat for a decade, the overdose death rate among U.S. adolescents nearly doubled from 2019 to 2020 — an alarming climb that continued into 2021, a study in JAMA shows. It’s not a surge of 14- to 18-year-olds using drugs, researchers said. If anything, survey data indicate that fewer teens experimented with drugs during the pandemic. Rather, a main factor is the supply of increasingly deadly drugs, one that has driven overall overdose deaths to more than 100,000 per year and has now trickled down to adolescents. What teens may think is an opioid painkiller or Xanax diverted from the legal supply is now more likely to be a counterfeit tablet containing fentanyl or similar synthetic opioids. “Drug use is becoming more dangerous, not more common” among adolescents, study co-author Joseph Friedman told STAT’s Andrew Joseph. Read more.
From the antibiotic overuse front, the American Journal of Preventive Medicine informs us “Unnecessary prescription of antibiotic prophylaxis by dentists continues to be common. Antimicrobial stewardship strategies are needed to improve prescribing by dentists.” No bueno.
Also in the no bueno department, Healthcare Dive calls our attention to a Lown Institute report
— U.S. nonprofit hospitals often get tax breaks worth far more than they spend on charity care and community investment, according to a new report from the Lown Institute. Prominent systems such as Providence, Trinity Health, Mass General Brigham and the Cleveland Clinic had some of the largest of these “fair share deficits,” the healthcare think tank said.
— The Lown Institute found 227 of the 275 hospital systems it studied spent less on charity care and community investment than the value of their tax exemptions. The fair share deficits of all hospital systems studied totaled $18.4 billion, which the institute argues could have been used to address health equity, housing, food insecurity and other local needs.
— Many of the hospital systems also received hundreds of millions of dollars in grant funding through the Coronavirus Aid, Relief, and Economic Security Act in 2020. The 275 systems examined operate more than 1,800 hospitals nationwide.
From the Rx coverage department. Drug Channel assesses the recent CMS Office of Actuaries report projecting U.S. healthcare spending.
The econowonks at the Centers for Medicare & Medicaid Services (CMS) recently released the latest projections for U.S. spending on healthcare. (See links below.) These data provide our first official look at post-pandemic U.S. healthcare spending.
As you will see below, outpatient prescription drugs dispensed by retail and mail pharmacies are projected to remain a small share (8.4%) of total U.S. healthcare spending. What’s more, taxpayers—via Medicare and Medicaid—will continue to crowd out the private insurance market. One bright spot: consumers will account for an ever-smaller share of drug spending.
Thus, the government actuaries expect that pharmaceuticals will not be the key driver of U.S. healthcare spending growth. Will someone tell our elected officials?
Here are tidbits that also follow up from stories in recent FEHBlog posts
- Fierce Healthcare reports that the bipartisan bill to control the price of insulin to consumers will “ensure that plans and PBMs ‘cannot collect rebates, which drive up drug costs at the point of sale, on insulins that roll prices back to 2006 or equivalent levels,’ the release said.”
- Healthcare Dive reports that part of the Administration’s efforts to control medical debt includes the following
The Biden administration laid out a four-point plan to reduce America’s medical debt on Monday, including having the HHS dig into how providers’ billing practices impact care access and affordability.
Under the plan announced by Vice President Kamala Harris, the HHS will request data from 2,000 providers on their bill collection practices, lawsuits against patients, financial assistance offerings, debt buying practices and more. The HHS will use this information in grant determinations, and to shape data and policy recommendations to the public.
The department will also share potential violations with enforcement agencies.The White House is also guiding federal agencies to stop using medical debt as an underwriting factor in credit programs where possible
- Health Payer Intelligence discusses AHIP’s comments on the CDC’s draft, revised opioid prescription guidelines.
Finally here are some OPM and USPS tidbits
- Govexec offers an interview with the Postmaster General Louis DeJoy.
- Federal News Network tells us
The Office of Personnel Management issued a second round of guidance to agencies on Tuesday, outlining several ways agencies should make employees more aware of their collective bargaining rights.
OPM’s guidance also directs agencies to quickly process requests to pay union dues through payroll deductions, and train managers and supervisors on how to remain neutral during union organizing campaigns.
OPM Director Kiran Ahuja, in a blog post, said the guidance is part of the administration’s focus on making the federal government a model employer in a competitive labor market.