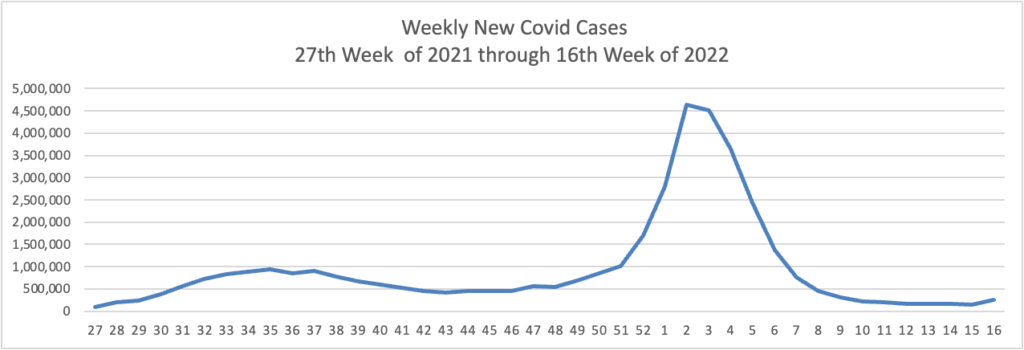
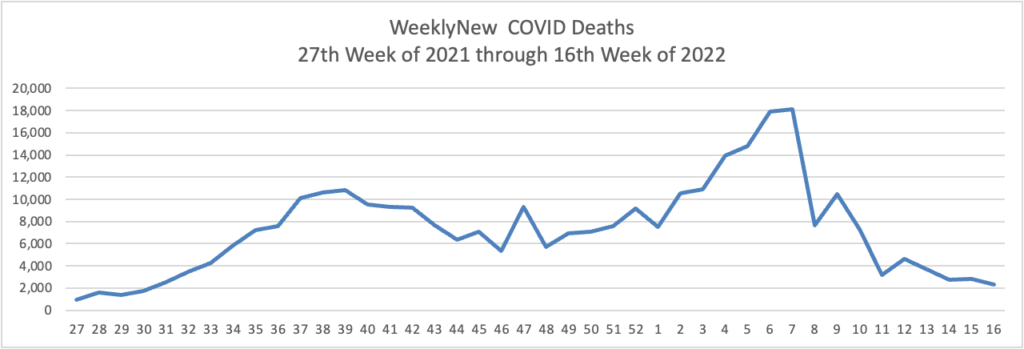
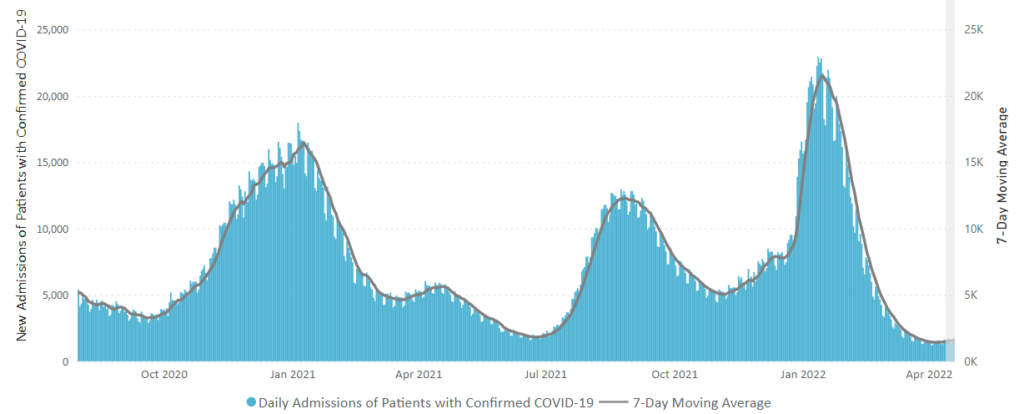
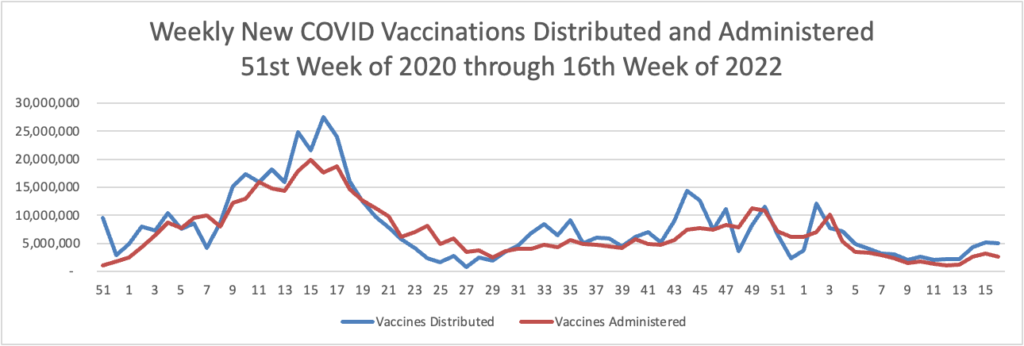
Weekend update

Congress returns from its two-week-long District / State work break tomorrow and resumes Committee business on Tuesday. The House of Representatives and the Senate also resume floor voting on Tuesday.
The Wall Street Journal adds
Once Congress returns to work, Democrats say they hope to get the bipartisan China competition bill signed into law. In the coming weeks, they are preparing to make a final attempt at resurrecting elements of the healthcare, education and climate package, which included provisions designed to lower the price of some prescription drugs. Sen. Joe Manchin (D., W.Va.), who scuttled the earlier bill in the Senate that all Republicans opposed, has said he could support a narrower package focused on climate and drug prices. * * *
Leaders of the center-left New Democrat Coalition urged the president in a recent meeting to work with Congress to focus on the China legislation and a narrow social spending and climate bill, said Rep. Suzan DelBene (D., Wash.), the group’s chair. In a separate meeting, the Congressional Progressive Caucus urged the president to use executive orders if legislation stalled, said CPC Chair Rep. Pramila Jayapal (D., Wash.).
Republicans say Democrats are out of touch with the electorate and that new spending will add to inflation. They also say that many of Democrats’ proposed actions could actually undercut them at the polls. If Republicans were to win back Congress, “the message to the president would be quit all the left-wing stuff, move to the center and work on things you can agree on,” said Senate Minority Leader Mitch McConnell (R., Ky.).
In other scheduling news, OPM and AHIP will be holding their annual FEHB carrier conference on Wednesday and Thursday this week. The conference will be held virtual. Hopefully next year, the conference will be held in person and a month earlier.
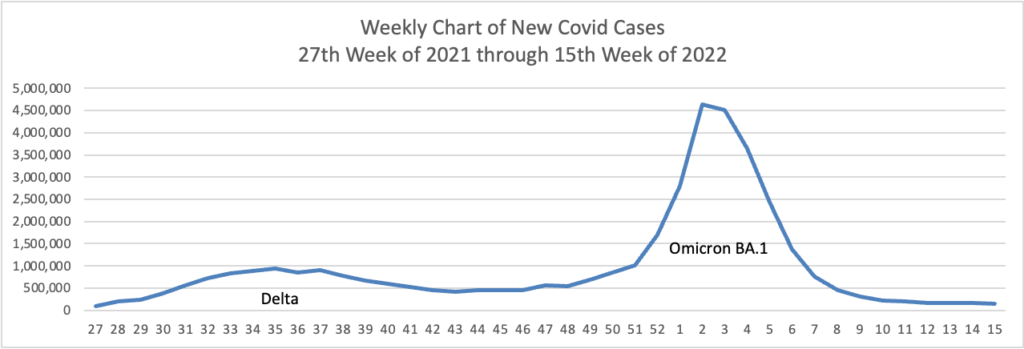
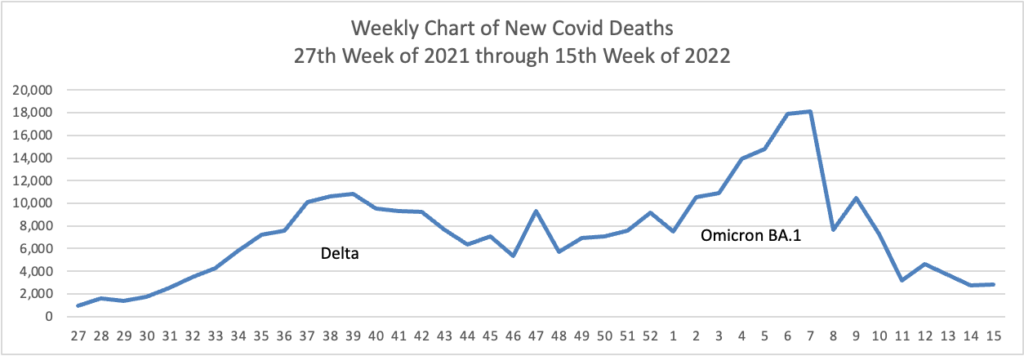
From the Omicron and siblings front, the American Medical Association offers what doctors wish their patients knew about the Omicron BA.2 subvariant. Nancy Crum, MD, an infectious disease specialist at Avita Health System in Galion, Ohio explained
We’ve been seeing a lot more of sore throat and pharyngitis that we didn’t really see before,” said Dr. Crum. Some of the other symptoms experienced are “very similar to the other coronaviruses such as febrile illness and respiratory symptoms.”
“Patients can also have gastrointestinal symptoms such as diarrhea, and loss of taste or loss of smell, although I’ve seen that a lot less with the newer variants,” she said, noting that symptoms for BA.2 may also include muscle or body aches, headache, nausea or vomiting, and congestion.
“We’re seeing very low rates of positivity for coronavirus right now and we’re actually seeing more influenza,” said Dr. Crum. That’s why “everyone coming in with any of those symptoms gets both a COVID test and an influenza test at the same time.”
Bloomberg’s Prognosis adds
The U.S. government is finishing plans to make Pfizer Inc.’s Covid-19 pill available at any pharmacy across the country, with supply increasing as the BA.2 sub-variant drives an uptick in cases and hospitalizations.
The administration will outline a plan [this coming] week aimed at getting the pill, Paxlovid, to additional people who’d otherwise face a more serious case of Covid-19, an administration official said Friday. The official asked not to be identified ahead of an announcement.
Use of oral antiviral pills in the U.S. jumped 103% between March 27 and April 10, the official said. The White House wants to drive that number higher, and signal to health providers to err on the side of prescribing the pills, rather than worrying about scarcity.
Let’s go.
STAT News advises
The World Health Organization said Saturday that 12 countries have reported at least 169 unusual cases of hepatitis in children, with 17 of the children having undergone liver transplants as a consequence. At least one child has died.
The WHO’s European division, which is taking the lead on the investigation into the mysterious outbreak, urged countries to look for, investigate, and report similar cases.
“Although the numbers aren’t big, the consequences have been quite severe,” Richard Pebody, who heads the high threats pathogen team at the WHO’s European division, told STAT in an interview. “It’s important that countries look.” * * *
The U.S. has seen 11 cases — nine in Alabama and two in North Carolina. The first Alabama cases date back to October and November of 2021, the earliest known cases. Pebody said most of the others are more recent. * * *
Suspicion has centered on an unexpected suspect — an adenovirus, specifically adenovirus type 41. At least 74 of the affected children have tested positive for adenovirus infection and molecular testing has turned up evidence of adenovirus 41 in 18 of those children.
Authorities have ruled out any possibility that Covid vaccines might have been involved in these cases. The vast majority of the children were not vaccinated, the WHO statement said.