Thursday Miscellany

From Capitol Hill, Fierce Healthcare reports
Several bipartisan senators are clamoring for more transparency into how pharmacy benefit managers conduct their business, potentially foreshadowing action on legislation to require new disclosures for the industry.
A subcommittee of the Senate Commerce Committee held a hearing Thursday on PBMs and their role in the pharmaceutical marketplace. Senators claimed there is an absence of competition in the industry and potential conflicts of interest.
“PBMs are not the only cause of drug price inflation and excessive pricing, but they are integral to this system,” said Sen. Richard Blumenthal, D-Connecticut, the subcommittee’s chairman. “They are part of an increasingly integrated, uncompetitive system involving PBMs owned or owning insurers and constraining pharmacies in the amount of information that they give to consumers. That is one slice of a broken system.”
Healthcare Dive informs us
Members of the healthcare industry are once again pressuring Congress to remove what they say is a major pain point in their operations and in the delivery of patient care: the ban on a nationwide unique patient identifier.
Almost 120 health IT groups, EHR vendors, hospitals, physicians and health insurers sent letters on Wednesday to House and Senate appropriators urging them to remove decades-old rider language in a 2023 appropriations bill that prevents the HHS from spending federal dollars to create or adopt a UPI standard.
Signees, including payer lobby AHIP, software companies Cerner and Epic, and health systems Banner Health and Intermountain, called the ban “archaic” in the letters. However, regulators have noted a UPI is unlikely to be a silver bullet against the nation’s patient matching problem.
The patient identifier strikes the FEHBlog as a key to interoperability as well as improving patient safety. Fund it, Congress!
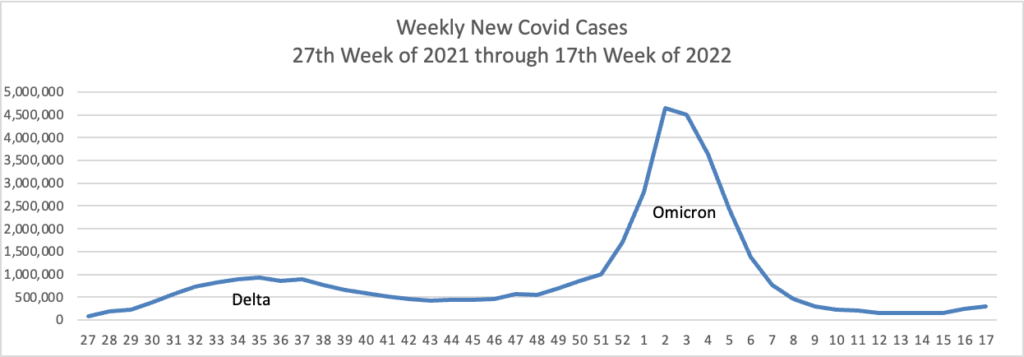
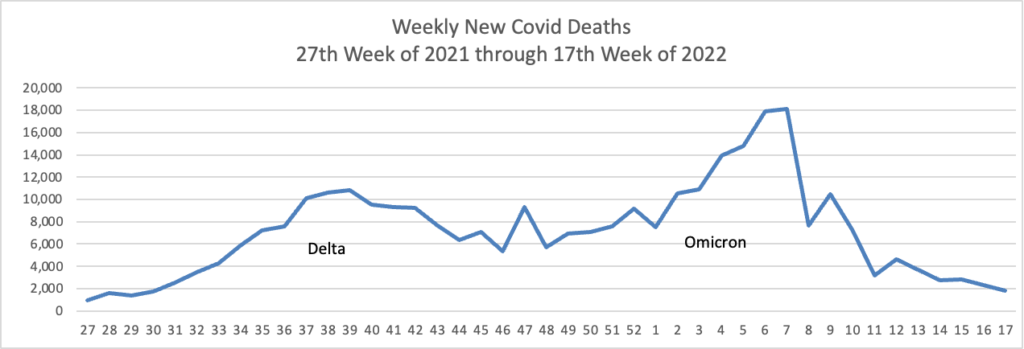
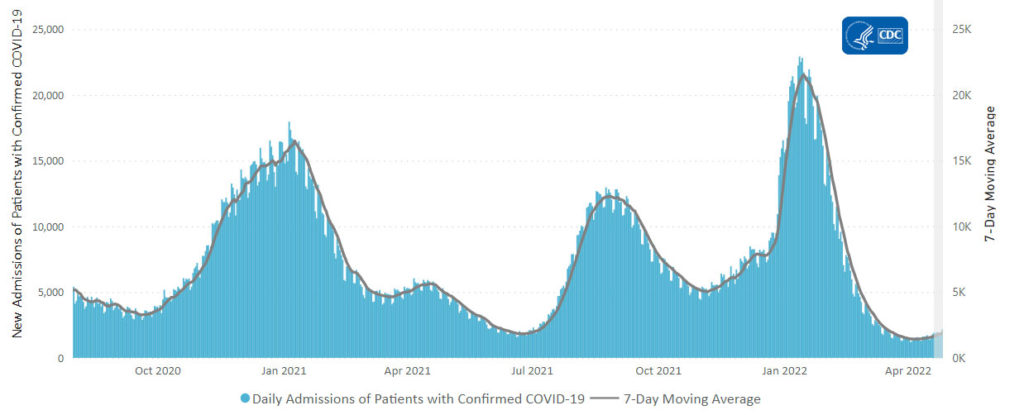
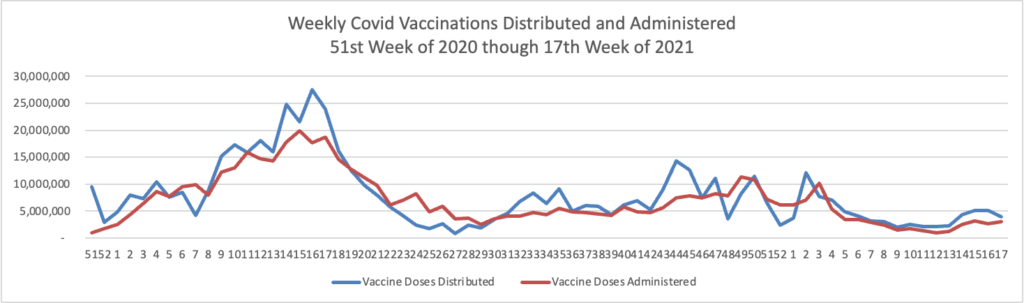
From the Omicron and siblings front, Medpage Today reports
Use of Johnson and Johnson’s (J&J) COVID-19 vaccine should only be limited to certain adults, the FDA said on Thursday.
Due to an updated analysis of the rare cases of thrombosis with thrombocytopenia syndrome (TTS), which typically occur 1 to 2 weeks after vaccination, use of the J&J vaccine should be restricted to those for whom mRNA vaccines are “not accessible or clinically appropriate,” or who would not get vaccinated if not for the J&J vaccine, the agency said.
It’s unfortunate that the only one-shot vaccine, which helped public health authorities reach underserved communities, is now knocked down for the mandatory eight count.
From the healthcare innovations front, Fierce Healthcare informs us
UnitedHealthcare has partnered with Kaia Health on a new virtual physical therapy program.
The program aims to offer 24/7, on-demand exercise feedback to eligible members with musculoskeletal conditions, the health insurance giant said. Members who are recovering from surgery or an injury will be asked to complete an assessment of current issues and will be referred to the program based on that assessment.
Eligible members will then be able to download Kaia’s app to access its physical therapy tools, which use artificial intelligence to support patients through physical therapy exercise and monitor progress.
Cigna is launching a new provider consult service that aims to improve outcomes for patients with cancer.
The program, backed by the capabilities of the insurer’s Evernorth subsidiary, allows community oncologists to connect with cancer subspecialty experts at centers designated by the National Cancer Institute (NCI). These connections will allow patients to benefit from the latest innovations in cancer care while also keeping their care close to home, Cigna said.
In a pilot, community oncologists had their treatment plans reviewed by experts and in 40% of cases reviewed, patients were recommended alternative tests or treatment based on new advancements in research.
Also a ZDNet reporter discusses his experience of wearing a continuous glucose monitor for 40 days.
I learned a lot about my body and how it reacts, and that’s information I can and do still use on a daily basis even if I don’t have an app yelling at me. Since I stopped wearing the Signos and getting insight, I’ve stuck to a healthier diet and routine exercise.
Good read.
From the healthcare business front, Healthcare Dive tells us
Centene said Thursday it has inked separate agreements to sell two of its pharmacy businesses in deals totaling $2.8 billion.
The payer plans to sell Magellan Rx to Prime Therapeutics and Pantherx to The Vistria Group, General Atlantic and Nautic Partners.
The deals are subject to regulatory approval. Magellan Rx is expected to close in the fourth quarter while Pantherx is anticipated to close in the next two to four months.
Thursday’s news builds on Centene’s plan to sell off non-core assets as it looks to sharpen its focus on its main [health insurance] business.
From the federal employment front, Govexec identifies the agencies who scored best and worst on the key employee morale questions of the recently released OPM 2021 Federal Employee Viewpoint Survey.
The National Science Foundation, Federal Energy Regulatory Commission, General Services Administration and the Pension Benefit Guaranty Corporation each landed in the top five on questions related to employees’ job satisfaction, senior leaders’ ability to motivate the workforce, and whether employees believe their agency will use Federal Employee Viewpoint Survey results to improve the workplace.
On the other hand, four agencies found themselves consistently near the bottom on these same questions. The Homeland Security Department, Social Security Administration, as well as the Justice and State departments all found themselves in the bottom five of at least two of these three questions.