Tuesday’s Tidbits

From Capitol Hill, STAT News reports
The sweeping FDA funding bill unveiled by senators Friday would direct the FDA to stand up an “intra-agency coordinating council” to oversee its use of the controversial accelerated approval pathway, my colleague Lev Facher writes. The policy seems a clear response to the controversy over the FDA’s approval of Aduhelm — and it’s the latest sign that lawmakers are within striking distance of reforming the controversial pathway, which allows the FDA to greenlight drugs without clear evidence they extend patients’ lives.
Accelerated approval not your bailiwick? There’s plenty more in the 433-page bill too, including a proposal from Sen. Elizabeth Warren that would force the FDA to finally release regulations meant to lower the cost of hearing aids. Conspicuously missing from the legislation? Any of the House’s proposals on clinical trial diversity.
For more on the package, check out Lev’s story here.
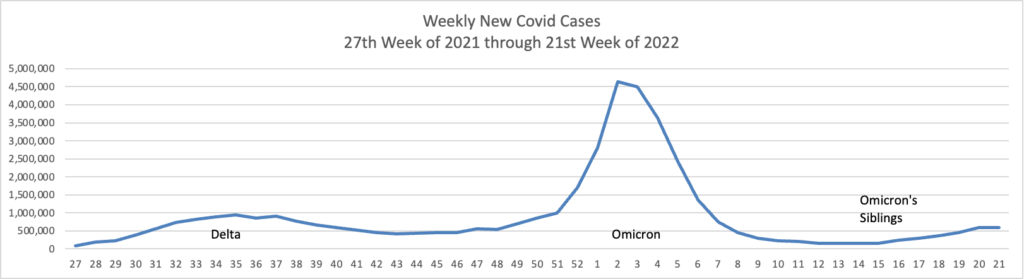
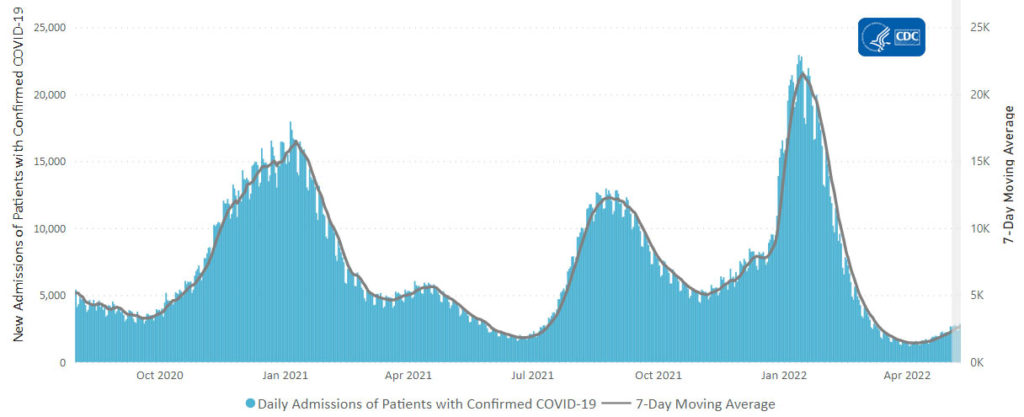
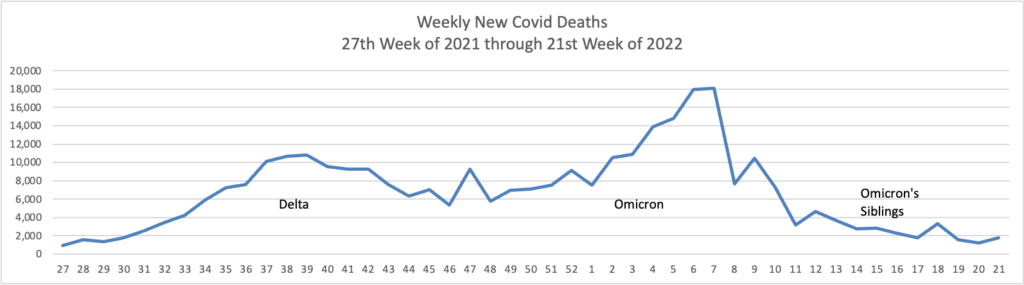
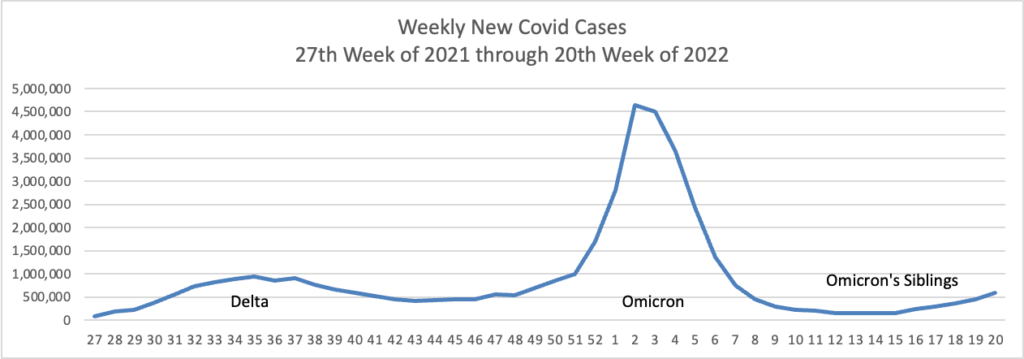
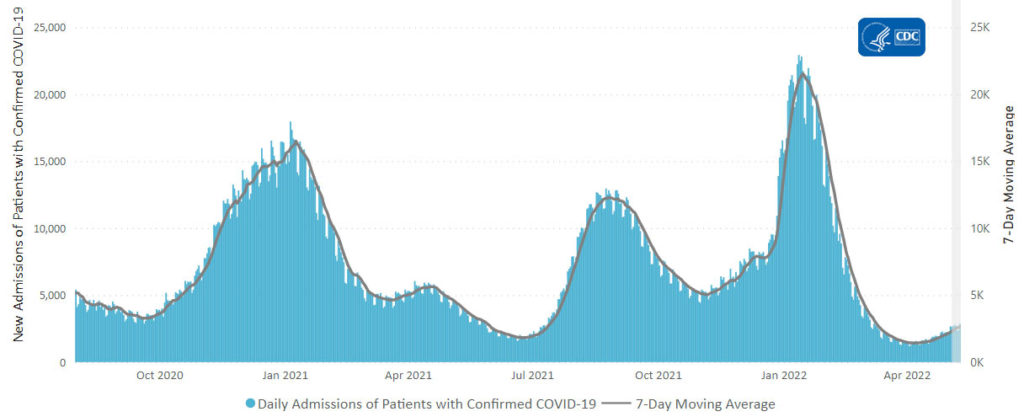
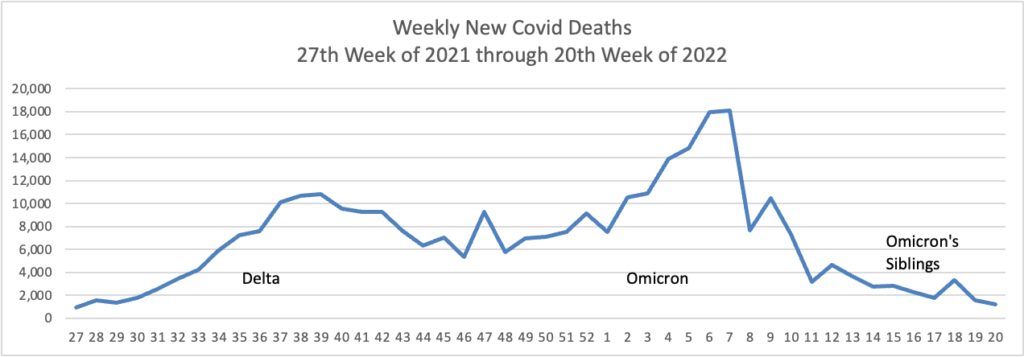
From the Omicron and siblings front
AP tells us
The coronavirus mutant [BA 2.12.1] that is now dominant in the United States is a member of the omicron family but scientists say it spreads faster than its omicron predecessors, is adept at escaping immunity and might possibly cause more serious disease.
Why? Because it combines properties of both omicron and delta, the nation’s dominant variant in the middle of last year.
A genetic trait that harkens back to the pandemic’s past, known as a “delta mutation,” appears to allow the virus “to escape pre-existing immunity from vaccination and prior infection, especially if you were infected in the omicron wave,” said Dr. Wesley Long, a pathologist at Houston Methodist in Texas. That’s because the original omicron strain that swept the world didn’t have the mutation.
Tricky little devil.
The Wall Street Journal adds that
Most people with Covid-19 will still test positive on a rapid test at five days, and a “fairly large percentage” test positive after 10 days, CDC spokeswoman Jasmine Reed said. Infectiousness drops significantly at eight days, with few people remaining contagious at 10 days, she said.
The CDC guidance also takes symptoms into account as a factor to gauge contagiousness, she said, noting that people should only leave isolation after five days if their symptoms are improving. The CDC recommends that everyone wear a well-fitted mask and avoid travel and being around high-risk people for 10 days, no matter when the person leaves isolation. * * *
At the beginning of an infection, when a person’s viral load is rising, it might take a few days before tests turn positive. That is why health authorities recommend that people with symptoms and negative rapid-test results wait and retest or get a more sensitive lab-based PCR test.
As a person’s viral load drops, rapid tests are a better indicator of who is no longer infectious, public-health experts said. * * *
Immunocompromised people and those who get severely ill can be contagious even longer than 10 days, studies suggest, and patients who experience rebounding symptoms or who test positive again after taking Pfizer Inc.’s Paxlovid pill should also assume that they are contagious, infectious-disease experts said.
David Leonhardt writes about the benefits of one-way masking in his New York Times column today. “Because masks work and mandates often don’t, people can make their own decisions. Anybody who wants to wear a snug, high-quality mask can do so and will be less likely to contract Covid.” That makes sense to the FEHBlog.
In healthcare business new, the American Hospital Association informs us
According to a report by Kaufman Hall, hospitals faced decreases in both patient volume and revenue in April. Year-to-date hospitals have struggled to rebound from winter surges and new spikes in COVID-19. Median operating margins declined for the fourth straight month with patient volume down 6.5%, revenue down 7%, and expenses continued to climb well above pre-pandemic levels and 9.6% year-to-date higher than 2021.
Healthcare Dive reports
* In its latest earnings report, Change Healthcare highlighted its momentum heading into the next fiscal year, outlining what 2023 could look like for the data analytics company as a standalone business in the event its controversial $13 billion merger with UnitedHealth falls through.
* In previous quarters, Change has not provided formal forward-looking guidance given the pending transaction. But in fourth-quarter results released last week, Change said it expects between 2% to 4% growth in its core Solutions business in 2023, moderately below analysts’ consensus, and roughly flat earnings before interest, taxes, depreciation and amortization margins.
* Change noted the flat margins are due to some wage pressure, lower volume-driven revenues as COVID-19 cases drop and client attrition due to its proposed merger with UnitedHealth’s health services arm, Optum.
The trial over the Justice Department’s challenge to this merger begins in August.
In public health news Medscape explains
Each day between 2017 and 2019, nearly 2,300 adolescents and young adults became new daily tobacco users — a figure that mirrors statistics from 1989 to 1993.
According to a longitudinal study, the total number of daily vape (or e-cigarette) users under 21 years of age rose to more than 1 million by 2019. Of those, 56.3% used Juul products in particular, reported cancer prevention researcher John Pierce, PhD, of the University of California San Diego in La Jolla, and colleagues in Pediatrics.
“The large increase in daily use among U.S. adolescents could presage future health consequences and needs urgent additional action from the [FDA],” the authors wrote.
From the mental health coverage front, the Society for Human Resources Management discusses how employers are grappling with a surge in employee mental health issues.
Also the Biden Administration marks the end of mental health awareness month by listing its accomplishments and plans, including
In addition to facilitating access to comprehensive telebehavioral health benefits through the Federal Employees Health Benefits Program, the Office of Personnel Management (OPM) is working to reinvigorate Employee Assistance Programs provided by all federal agencies to better meet employees’ behavioral health needs, while disseminating best practices and new ideas for improving federal workplace mental health.
From the medical research front, STAT News reports
Bay Area biotech Ultima Genomics on Tuesday claimed that its technology can sequence a whole human genome for $100, making it a surprise player in the race to read DNA quickly, accurately, and cheaply.
The company didn’t provide specifics or immediately reply to an inquiry from STAT as to how it calculated that cost. But a $100 genome would represent a major drop in price, one that could help researchers unlock sequencing’s potential to unravel the mysteries of undiagnosed diseases, spot early signs of cancer, and better understand human health. That’s because while the cost of reading a full human genome has plummeted from around $95 million in 2001 to about $560 in 2021, according to the National Human Genome Research Institute, sequencing is still too pricey to be routinely used in health care and research.
That’s an intriguing development.