Weekend update

From the Capitol Hill front, the House of Representatives and the Senate will be in session this week for floor voting and Committee business.
Roll Call reports
A bipartisan group of senators announced an agreement Sunday on significant updates to the nation’s gun laws, and the Senate majority leader said it would be put on the floor once legislative text is ready.
The agreement, announced by 10 Republicans and 10 Democratic caucus members, certainly will not go as far as many Democrats would have hoped, but the scale of the GOP support suggests it could get the all-important 60 votes to overcome the filibuster rule that kept derailing the last bipartisan attempt to change gun laws, in 2013.
The agreement, which is not yet in legislative language, is the product of discussions led by Sens. Christopher S. Murphy, D-Conn., and John Cornyn, R-Texas, in the wake of recent mass shootings, including at an elementary school in Uvalde, Texas.
“Our plan increases needed mental health resources, improves school safety and support for students, and helps ensure dangerous criminals and those who are adjudicated as mentally ill can’t purchase weapons,” the 20 senators said in a statement. “Most importantly, our plan saves lives while also protecting the constitutional rights of law-abiding Americans.”
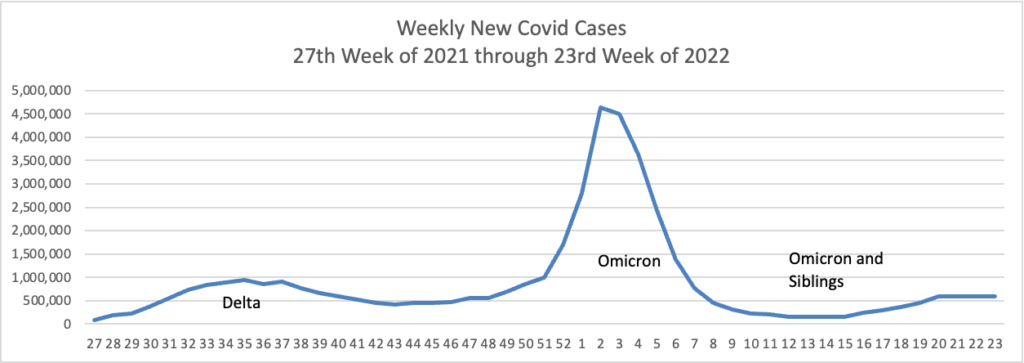
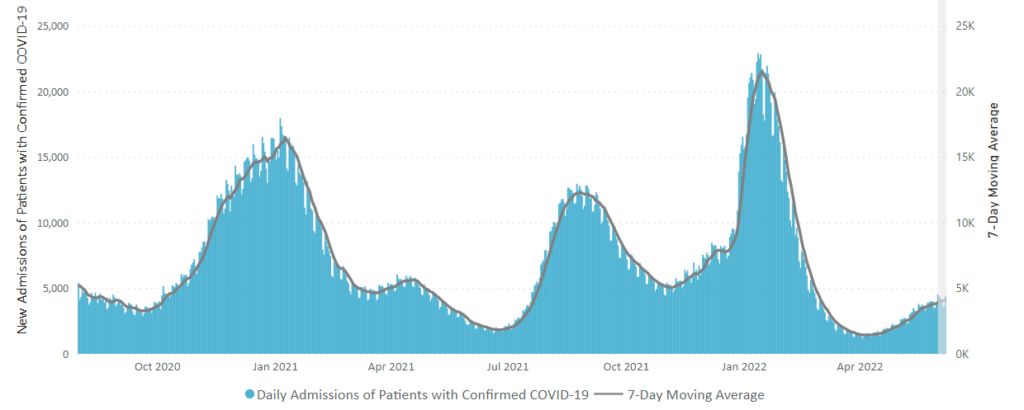
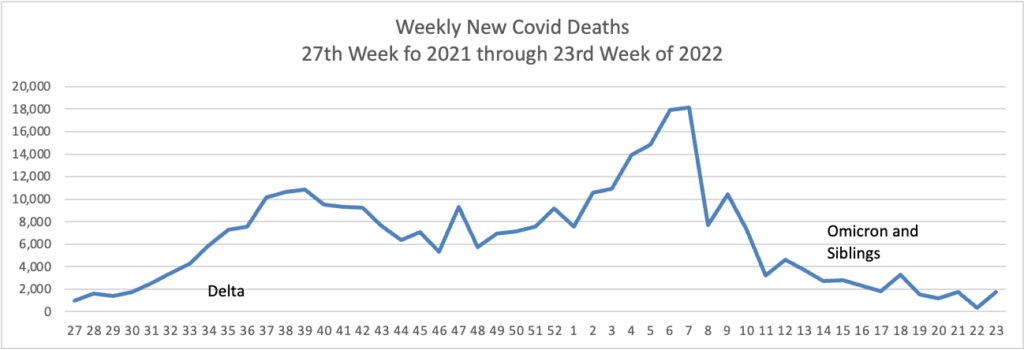
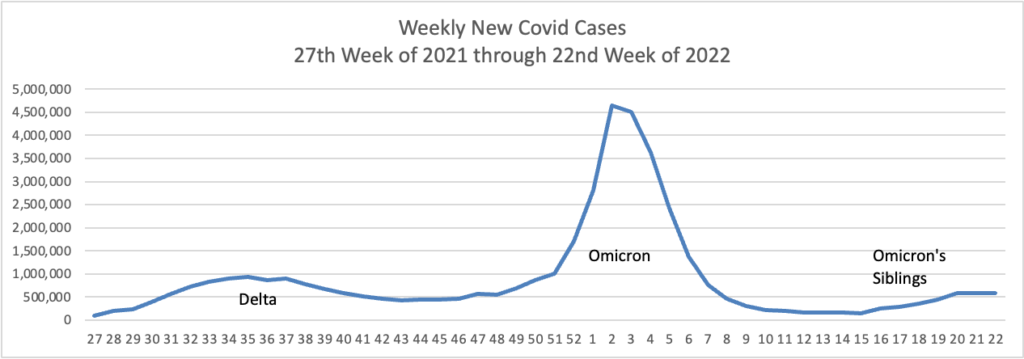
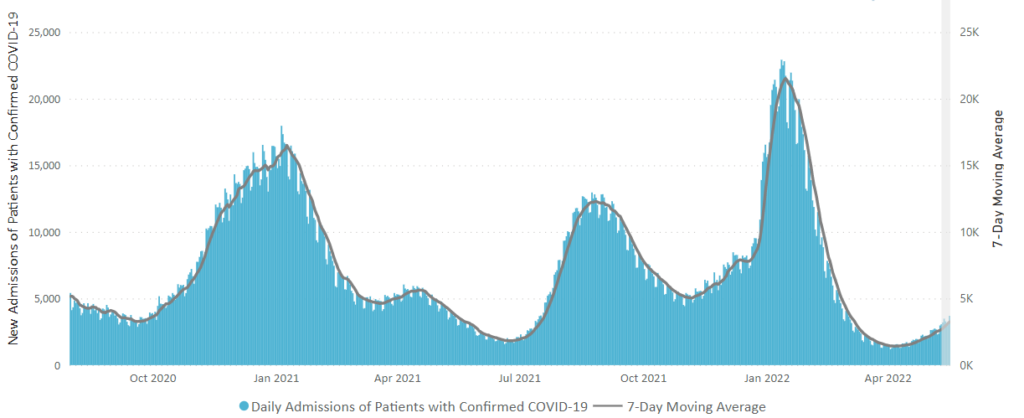
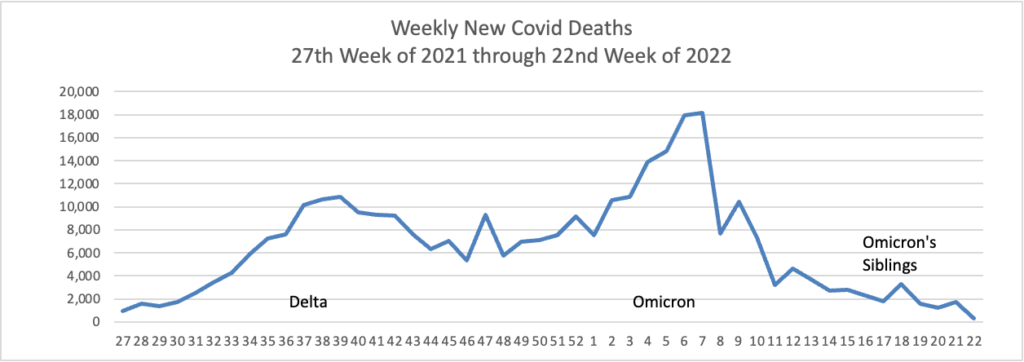
From the Omicron and siblings front —
MedPage Today discusses the latest Omicron siblings — BA.4 and BA.5. “Generally, BA.4 and BA.5 variants cause mild disease but spread in large numbers potentially because, unlike the Wuhan strain, which settles in the lungs, these newer strains seem to attach to the more benign upper nasal passages.”
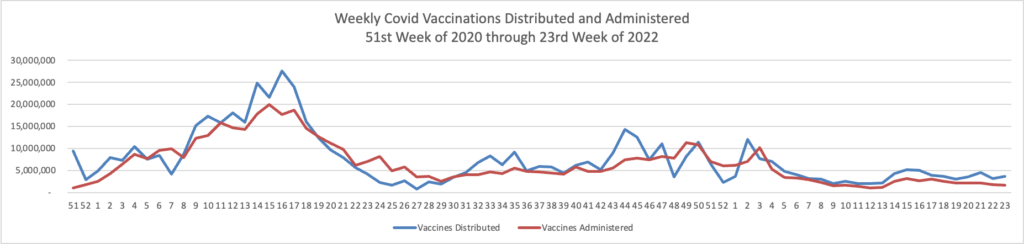
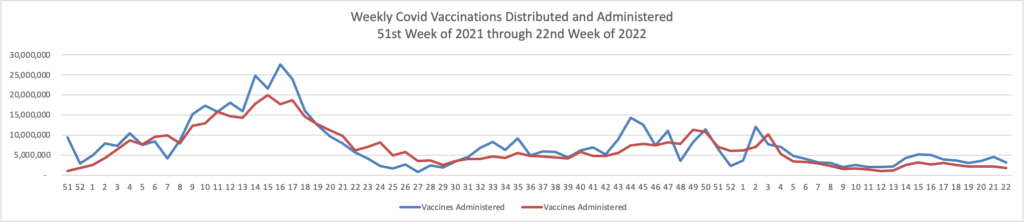
Bloomberg Prognosis answers a reader’s question about whether to get a fourth Covid booster now.
Moderna said just this week that a new version of its Covid vaccine led to a better antibody response against omicron compared with its current mRNA shot. It plans to submit data to the US Food and Drug Administration in the coming weeks and hopes the shot will be available as soon as late summer. That shot is what’s called a bivalent vaccine, meaning it contains mRNA coding for the spike protein of both the original strain of the virus and omicron.
Pfizer should also have updated mRNA vaccines available as soon as the fall, says Monica Gandhi, an infectious disease expert at the University of California, San Francisco. The FDA will hold an advisory committee meeting later this month to address whether fall shots should be modified, and if so what strains they should include.
But while currently available boosters are less effective against omicron, Gandhi points out, they still do offer some protection. So, she says, that answer to whether to get boosted now depends on a few factors.
”I would advise — depending on case rates in your area and your age— getting the fourth dose now,” she says. “And then deciding what to get in the fall.”
MedPage Today also explains why “the [Novovax Covid] technology is quite innovative and has potential to enhance protection against” the disease.
From the healthcare business front, the Wall Street Journal reports on a
A philanthropic organization founded by the ex-energy trader [John Arnold] and his wife Laura is providing financial backing to three lawsuits against giant hospital systems in Wisconsin, Connecticut and North Carolina, alleging the systems used their market power to squash competition and illegally inflate prices. The systems say the lawsuits from employers and consumers are baseless. * * *
The financing from Arnold Ventures is supporting the work of Fairmark Partners LLP, the law firm behind the lawsuits. Fairmark secured its funding from the philanthropy after filing its first lawsuit, one of the founders said. The firm’s founders say the backing is key to a targeted effort to reshape hospital markets through the courts, and that it received money from other philanthropies it declined to identify.
While the FEHBlog is not a fan of litigation, this strikes him as a worthy effort.
From the medical research front —
- Kaiser Health News summarizes recent drug research developments.
- NPR Shots reports on efforts to connect human nerve systems to prosthetic devices.
- Fierce Healthcare tells us
Researchers at CVS studied the relationship between total cost of care and the use of National Comprehensive Cancer Network (NCCN) guidelines to direct care and found savings among both breast cancer and colon cancer patients. The studies, released at the American Society of Clinical Oncology’s meeting earlier this month, build on a similar analysis among lung cancer patients.
In both studies, the researchers found that using NCCN guidelines drove significant declines in total cost of care.
“Evidence-based medicine does result in improvement in quality of life,” Shirisha Reddy, M.D., senior medical director at CVS Health and an author on both studies, told Fierce Healthcare. “There’s a lot of external data that supports that.”
The first study included 937 patients with colon cancer. Among Medicare beneficiaries, concordance with NCCN guidelines was linked to a 33% reduction in total cost per care per member per month. The results were less significant among commercially insured patients.
In the second study, the researchers retrospectively looked at 315 patients with breast cancer. They found total cost of care reductions for patients treated in ways consistent with NCCN across multiple insurance types, including 25% among fully insured commercial patients, 28% among self-insured commercial patients and 43% in Medicare.
This included notable decreases in administered chemotherapy spend as well as outpatient care between Jan. 1, 2019, and Dec. 31, 2020.