Midweek Update

From Capitol Hill, Fierce Healthcare reports
Senate Democrats have narrowly reached a deal on legislation to give Medicare the power to negotiate for lower drug prices.
The Senate released text Wednesday (PDF) on the deal that also repeals the controversial Part D rebate rule and installs a cap on monthly cost-sharing payments for Part D and Medicare Advantage plans.
The legislative text shows that starting in 2026, the Department of Health and Human Services will choose 10 drugs eligible for negotiation. The next year, the number of eligible drugs will increase to 15, and in 2029 and every year after by 20.
The sole-source drugs subject to negotiation will be chosen based in part on their total spending under Medicare Parts B and D. There is an exception for small biotech drugs from 2026 through 2028 such as vaccines and excludes certain orphan drugs as well.
Roll Call adds “Congress is fast approaching its scheduled August recess, followed by peak campaign season, so Democratic lawmakers only have a few more weeks in session to push their legislative priorities before they could lose control of either chamber in November.”
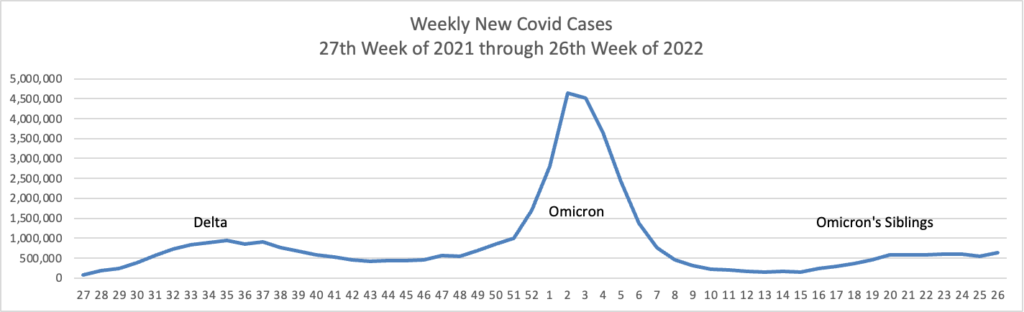
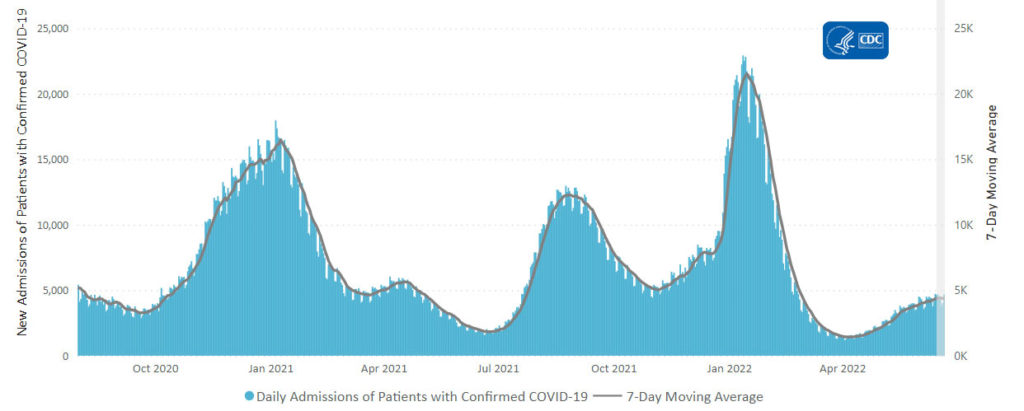
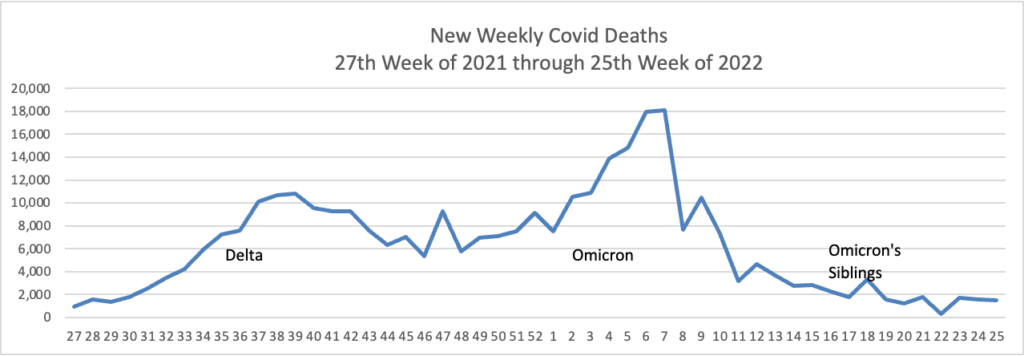
From the Omicron and siblings front, the American Hospital Association tells us
The Food and Drug Administration today authorized state-licensed pharmacists to prescribe Paxlovid (nirmatrelvir and ritonavir) to patients as a treatment for those at high risk of severe COVID-19. Because Paxlovid must be taken within five days of symptom onset, the change could spur expanded access and more-timely treatment of eligible patients. The change was made through an amended emergency use authorization.
This standing order approach should accelerate the continuing rollout of test to treat locations.
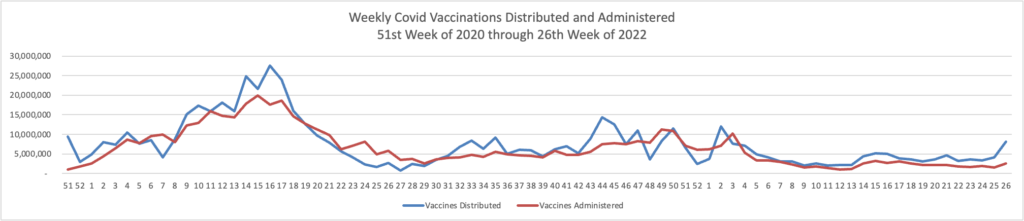
Regrettably the Wall Street Journal adds
Governments, drugmakers and vaccination sites are discarding tens of millions of unused Covid-19 vaccine doses amid sagging demand, a sharp reversal from the early days of the mass-vaccination campaign, when doses were scarce. * * *
In the U.S., about 90.6 million Covid-19 doses have been wasted, or 11.9% of the more than 762 million Covid-19 vaccine doses delivered since the shots became available in late 2020, according to the Centers for Disease Control and Prevention.
The wastage rate has accelerated recently: Some 12 million of the discarded doses have been thrown out since late May.
The disposals come during a significant drop in demand for Covid-19 vaccines, even with young children recently becoming eligible. The seven-day moving average of doses administered daily in the U.S. was about 155,000 as of June 21, down from about 1.1 million on Jan. 1 and the peak of about 3.5 million daily in April 2021.
Partly driving the wastage, health experts said, is the way the Covid-19 vaccines are packaged in multiuse vials containing from five to 20 doses. Once opened, the vials generally must be used within about 12 hours of opening or the remaining doses discarded.
From the telehealth front
Healthcare Dive reports
COVID-19 made its way back into the top five telehealth diagnoses nationally on Fair Health’s monthly tracker in April for the first time since January, according to the report out Wednesday.
Every U.S. census region except the South saw COVID-19 return to the top five diagnoses list, and the uptick is in line with rising cases reported in April by the Centers for Disease Control and Prevention.
Telehealth use overall also rose nationally and in every region after two months of decline, the report found.
Fierce Healthcare informs us
Teladoc is further building out its primary care offering, Primary360, with new services that enhance care coordination and grow in-home options.
Primary360 will now provide care coordination support and health plan in-network referrals alongside free same-day medication delivery from Capsule and in-home, on-demand phlebotomy services backed by Scarlet Health, according to an announcement Wednesday from Teladoc.
The new care coordination capabilities will allow Primary360’s care team to take a “holistic” view of the patient’s coverage and make streamlined referrals to Teladoc services they can access. The care team can also then ensure a patient is referred to an in-network provider when in-person services are necessary.
mHealth Intelligence reports “The burgeoning mental health epidemic in America is widespread across age groups, but the youth have faced a particularly challenging time amid the COVID-19 pandemic. As the youth mental health crisis reaches new heights, providers are increasingly turning to telehealth to help expand access to behavioral healthcare.”
In the same spirit, Health Data Management discusses best practices for hospitals interested in providing acute care at home services.
From the U.S healthcare front, Beckers Hospital Review calls our attention to the fact that “Money, formerly Money Magazine, and Leapfrog Group collaborated for their first shared ranking of “best hospitals” to help consumers make decisions about which healthcare institutions are best for their money. The inaugural list was released July 6 and can be found in full here.” Check it out.
From the fraud, waste and abuse front, Healthcare Dive reports
The federal government won or negotiated more than $5 billion in healthcare fraud judgments and settlements in its 2021 fiscal year, the largest amount ever in the history of the HHS and Department of Justice’s fraud and abuse enforcement program.
Due to those and other efforts from previous years, the government clawed back almost $1.9 billion, according to a new report from the departments.
Of that $1.9 billion, about $1.2 billion went to the Medicare trust funds, which are on increasingly precarious financial footing due to growing stress on the insurance program. In addition, roughly $99 million in federal Medicaid money was transferred back to the CMS.
Finally, Govexec brings us up to date on projections for 2023 annual raises for federal employees.
President Biden and House appropriators seem thus far to be in agreement that federal employees should receive an average 4.6% pay raise next year, but there are still several steps officials must take before it can be implemented at the end of the year. * * *
On Capitol Hill, there are still a few opportunities for federal employee groups and some lawmakers to try to increase the raise to the average 5.1% figure they have been advocating for.