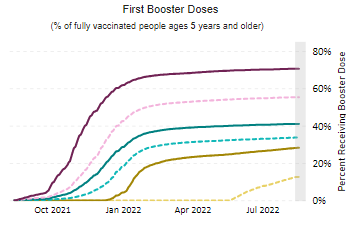
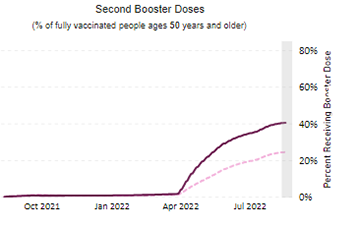
Friday Stats and More
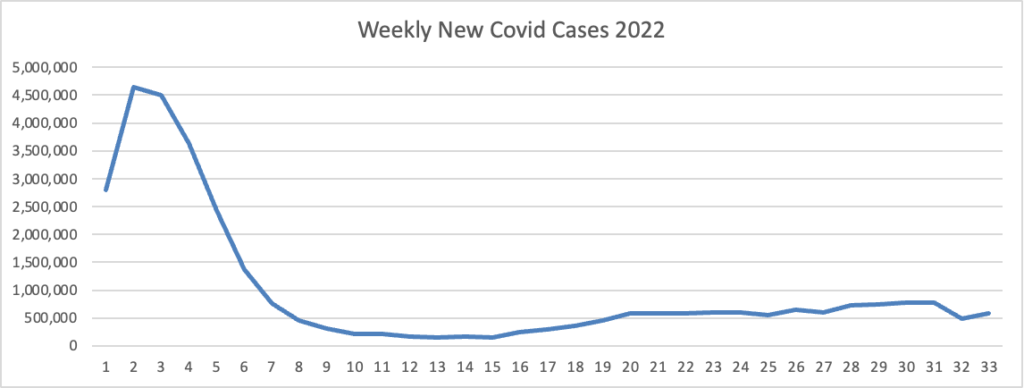
Based on the Centers for Disease Control’s Covid Data Tracker and using Thursday as the first day of the week, here is the FEHBlog’s latest weekly chart of new Covid cases for this year.
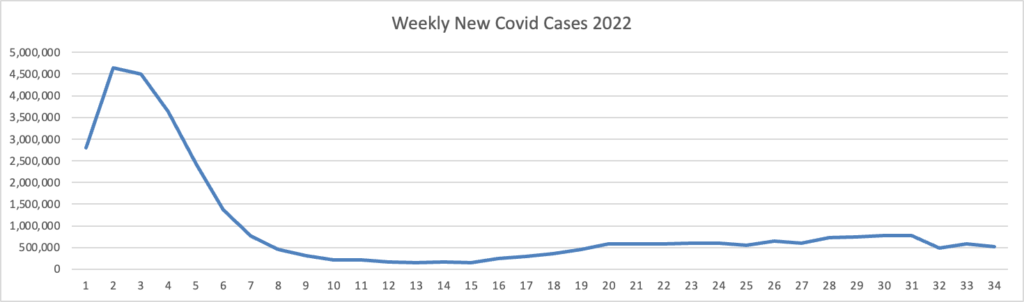
On the left side of the chart are the peak and downslope of the original Omincron strain, and what a peak it was. On the right side of the chart is the Omicron sibling’s plateau.
The CDC’s weekly review of its Covid statistics adds
As of August 24, 2022, the current 7-day moving average of daily new cases (90,676) decreased 6.7% compared with the previous 7-day moving average (97,184). * * *
CDC Nowcast projections* for the week ending August 27, 2022, estimate that the combined national proportion of lineages designated as Omicron will continue to be 100% with the predominant Omicron lineage being BA.5, projected at 88.7% (95% PI 87.3-89.8%).
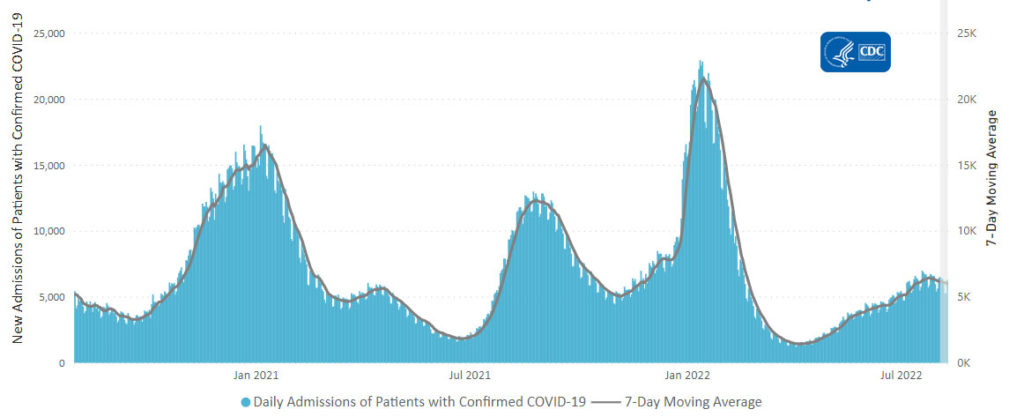
Here’s the CDC’s latest chart of daily trends in new Covid hospitalizations:
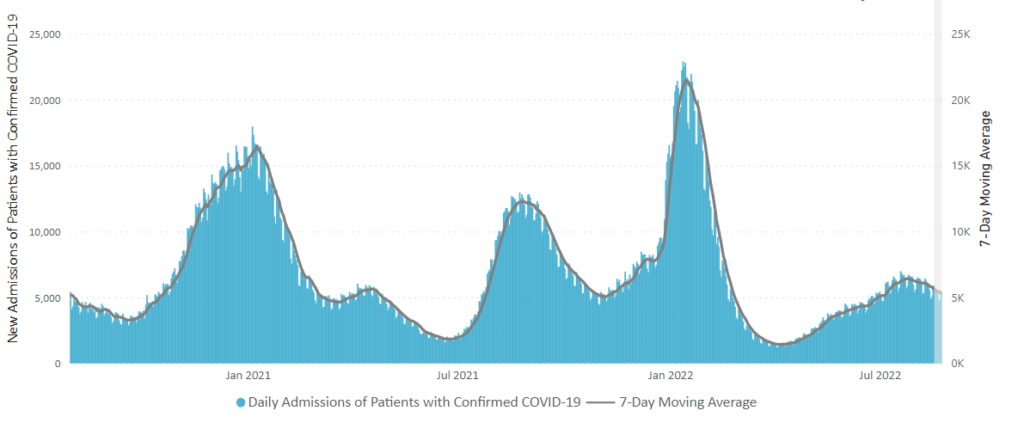
The CDC’s weekly review adds “The current 7-day daily average for August 17–23, 2022, was 5,314. This is a 6.6% decrease from the prior 7-day average (5,687) from August 10–16, 2022.” That converts to a very low percentage of new Covid cases.
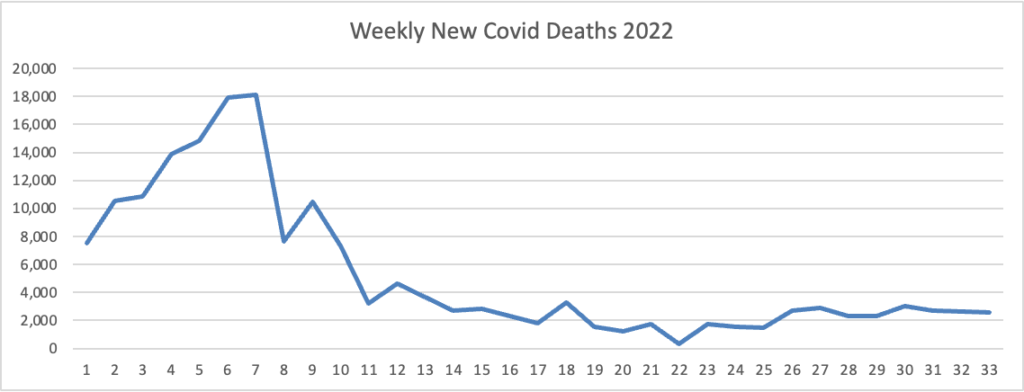
Here is the FEHBlog latest weekly chart of new Covid deaths for 2022:
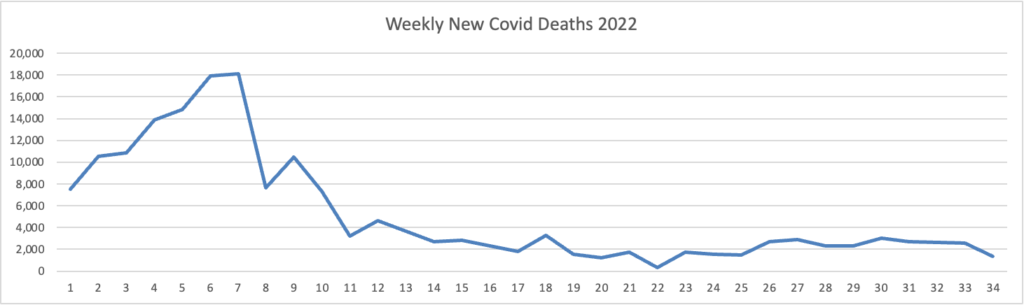
Omicron is on the left, and Omicron’s siblings are on the right. The CDC’s weekly review adds “The current 7-day moving average of new deaths (390) has decreased 11.6% compared with the previous 7-day moving average (441).”
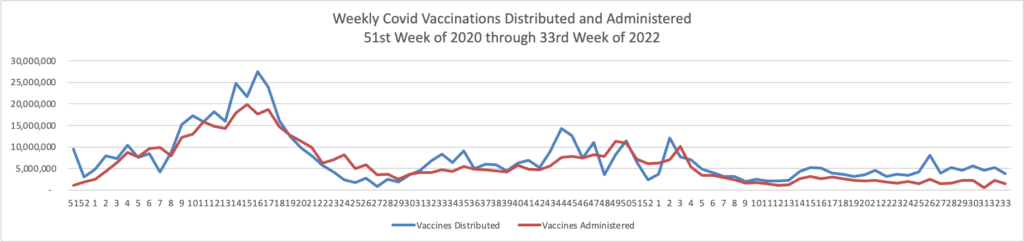
Contributing to the current low Covid death rate are the Covid vaccines, and here is the FEHBlog’s weekly chart of Covid vaccinations distributed and administered from the beginning of the Covid vaccination era in the 51st week of 2020 through the 34th week of 2022:
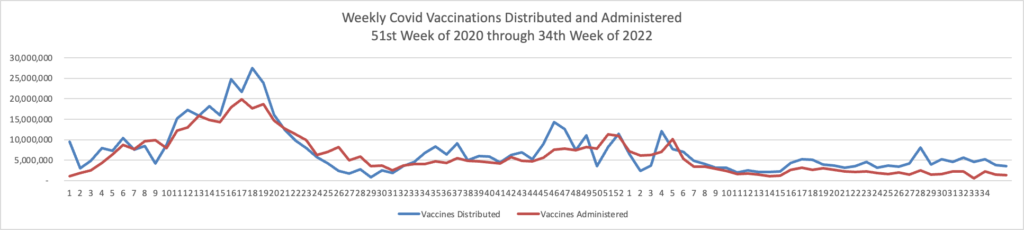
The CDC’s weekly review adds
As of August 24, 2022, 608.9 million vaccine doses have been administered in the United States. Overall, about 262.6 million people, or 79.1% of the total U.S. population, have received at least one dose of vaccine. About 223.9 million people, or 67.4% of the total U.S. population, have been fully vaccinated.
In other Covid news
- Medscape reports “Long COVID Mimics Other Post-Viral Conditions.” That’s reassuring.
- Medscape also tells us that a major weak spot on the Omicron variants which could lead to effective treatments.
In Covid-related legal news, STAT News informs us
It’s the stuff that headline writers’ dreams are made of: Moderna is suing Pfizer and BioNTech over their Covid vaccines.
As someone who has written dozens of catchy headlines about patent suits and read hundreds more, let me offer a bit of advice: Take several deep breaths. Most likely, this is less dramatic than it seems.
The reality of patent litigation in the pharmaceutical industry is that it proceeds at a glacial pace. And it rarely results in products being pulled from the market (Moderna isn’t even asking for that!) or for payments or royalties so significant that they dramatically change the profitability of a product. These lawsuits, though, can involve sums large enough that they make a financial difference to investors.
Time will tell.
From the monkeypox front, the New York Times reports
Monkeypox cases are declining in New York City and globally as more people get vaccinated and as they change their sexual behavior in response to the outbreak, health officials said this week.
New York City on Thursday reported that 2,885 monkeypox cases had been identified in the city since the first case in the city was identified in May. In mid-August, about 50 new monkeypox cases were being detected each day, a drop from the 70 or so new daily cases emerging in late July and early August, according to city data. * * *
Monkeypox infections are also declining in parts of California and in Europe, which at one point had 90 percent of the world’s cases. The World Health Organization on Thursday reported that monkeypox cases globally dropped 21 percent last week. But the overall trend masked rising cases in other parts of the world, including Latin America and Africa.
In New York, Dr. Vasan attributed the decline to the city’s efforts to get tens of thousands of people vaccinated; the city has administered 69,311 doses of the vaccine, according to city data.
In Medicare news, Fierce Healthcare tells us
More than 28 million people are in a Medicare Advantage (MA) plan in 2022, with the program accounting for nearly half of all Medicare beneficiaries, a new analysis finds.
The analysis, released Thursday by the Kaiser Family Foundation, also showed how spending on MA, especially on quality bonuses, has surged to take up more than half of all federal Medicare spending. The findings underscore the widespread interest in the insurance industry on the MA marketplace, which has grown more lucrative in recent years.
Kaiser found that the share of eligible Medicare beneficiaries who chose an MA plan has more than doubled since 2007, growing from 19% that year to 48% in 2022. An earlier projection from the Congressional Budget Office projected that the percentage of Medicare beneficiaries in MA will swell to 61% by 2032.
From the substance use disorder front, Health IT Analytics reports
A new study by Epic Research and the University of Maryland’s Center for Substance Abuse Research (CESAR) shows that only 5 percent of drug overdose patients admitted to the emergency department are tested for fentanyl and synthetic opioids, despite these drugs being the leading cause of death for Americans 18 to 45 years.
The Centers for Disease Control and Prevention (CDC) report that synthetic opioids are currently the main driver of drug overdose deaths, which increased by 31 percent from 2019 to 2020. Opioids were involved in 75 percent of all drug overdose deaths in 2020, and 82.3 percent of all opioid overdose deaths involved synthetic opioids.
That is confounding.
From the coding front, Becker’s Hospital CFO Review points out the top 10 states with the highest coding rates for social determinants of health diagnoses. The FEHBlog suspects that these states have the most reliable coders.