Thursday Miscellany

From Capitol Hill, Fierce Healthcare tells us
Republican lawmakers are pushing back on the Biden administration’s request for an additional $26 billion in COVID-19 and monkeypox funding, the AP, Politico and other news sources reported.
The White House’s ask came as Congress begins negotiations for a short-term funding bill needed to avoid a shutdown at the end of the month.
In comments to reporters, conservatives said their disapproval of additional pandemic spending was tied to inflation and concerns about how prior funds were used.
“The problem is they want to keep spending more money and throw more gasoline on the inflation fire,” Senator John Cornyn (R-Texas) told the AP. “I think that’s a bad idea.”
The lawmakers said that the White House should instead repurpose funds left over from earlier pandemic asks and begin transitioning the costs for certain efforts, such as vaccines, to the private sector or general public.
“There’s really no reason that the government should be paying for all of that,” Senator Roy Blunt (R-Mo) told the AP.
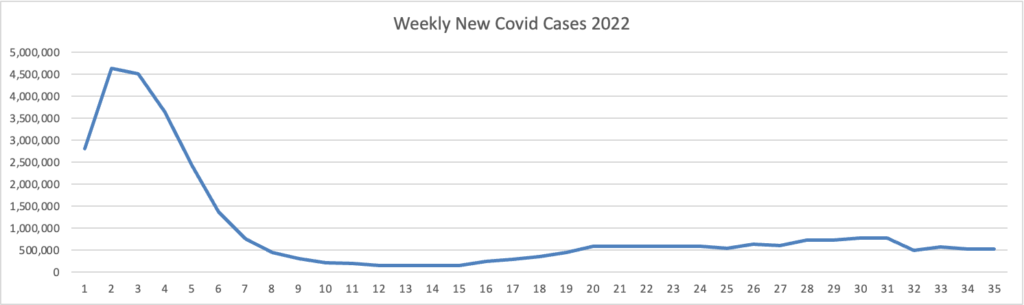
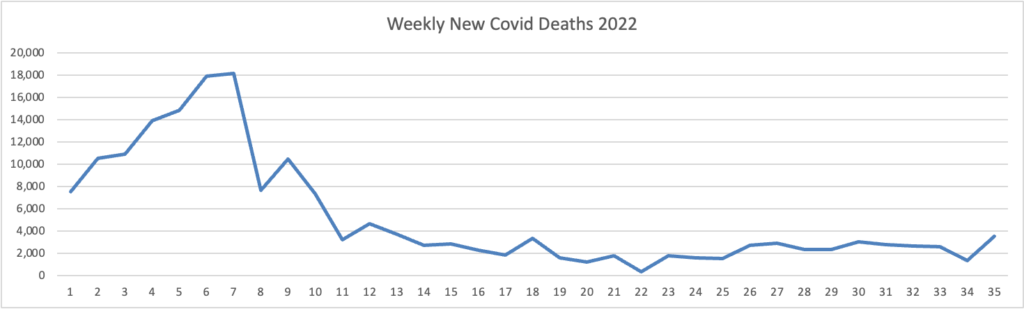
From the Omicron and siblings front —
Bloomberg discusses post Paxlovid rebound Covid.
Infectious disease specialists [writing in a recent New England Journal of Medicine report] say the rebounds aren’t uncommon, and patients should watch for them. They should also feel reassured that symptoms are almost always mild when a rebound occurs.
Because a rebounder can become contagious again, the Centers for Disease and Control and Prevention urges people to re-isolate and mask if symptoms recur and rapid tests turn positive again.
Specialists emphasize that though rebounds are an inconvenience, Paxlovid remains the treatment of choice for people at high risk of severe Covid.
Politico reports that doctors and Reuters reports that researchers are colloborating to understand and treat long Covid.
As the FEHBlog received his bivalent mRNA booster yesterday, it’s worth pointing out this advice from STAT News
If you’re planning to double up your new Covid booster with your annual flu shot in one visit this month, you might want to reconsider. Even though White House Covid coordinator Ashish Jha believes “this is why God gave us two arms — one for the flu shot and the other one for the Covid shot,” it’s still early to get a flu shot, STAT’s Helen Branswell warns. That’s because protection generated by flu vaccines erodes pretty quickly over the course of a flu season.
A flu vaccine dose given in early September may offer limited protection if the flu season doesn’t peak until February or even March, as it did during the unusually late 2021-2022 season. “You’ve got about four months of pretty solid protection,” Emily Martin, an epidemiologist who specializes in flu at the University of Michigan School of Public Health, told Helen. Read what other experts had to say.
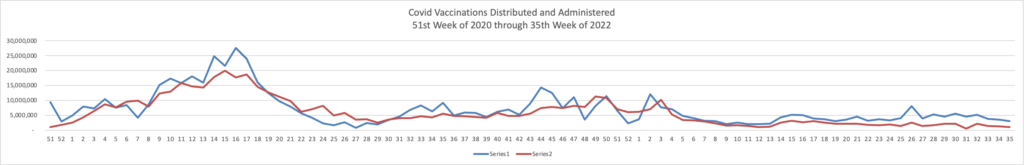
From the monkeypox front, CNBC informs us
* Demetre Daskalakis, a White House health official, said the monkeypox outbreak has slowed significantly since July as vaccination efforts have ramped up.
* The U.S. is still battling the largest monkeypox outbreak in the world with nearly 21,000 cases reported across all 50 states, Washington D.C. and Puerto Rico, according to CDC.
* The U.S. has administered more than 460,000 monkeypox vaccine doses so far, according to data from 35 states.
From the healthcare costs front, Health Payer Intelligence reports
Almost 83 cents of the average healthcare premium dollar goes towards prescription drugs and medical services, including inpatient and outpatient costs, emergency room costs, and doctor visits, according to research from AHIP.
AHIP analyzed data from commercial health insurance plans between 2018 and 2020 to determine how payers spend member premiums. The research reflects spending by employer-sponsored health plans and health plans in the individual market.
“This 3-year trend data includes the first year of the COVID-19 pandemic, when healthcare utilization was down dramatically as patients deferred care and isolated due to the risk of infection,” Matt Eyles, president and chief executive officer of AHIP, said in a press release.
Prescription drugs accounted for the largest portion of the premium dollar at 22.2 cents—up from 21.5 cents between 2016 and 2018. Prescription drug expenses include payments for outpatient prescription medications obtained from the pharmacy or medicines administered in a physician’s office or clinic.
The next largest chunk of the healthcare dollar (19.9 cents) went toward outpatient hospital costs. This includes physician and facility payments for treatment in the outpatient department of hospitals, such as receiving an X-ray or seeing a primary care physician. However, it does not include emergency room costs, such as payments for emergency room visits and ambulance transportation, which accounted for 3.3 cents of the premium dollar.
Inpatient costs represented 19 cents of the dollar, AHIP found. These expenses include payments for all services during hospitalization, including payments to physicians, facility payments, costs for prescription drug administration, and room and board costs.
Almost 12 cents per dollar went toward doctor visits, which included payments to physicians for services provided in an office, clinic, or urgent care facility. The 11.8 cents also went toward equipment and supplies used during a visit and nursing staff salaries.
The National Alliance of Healthcare Consumer Coalitions is offering at no charge its
playbook, “Beyond Hospital Transparency: Getting to Fair Price,” helps purchasers navigate and understand how to best leverage newly available hospital price and quality transparency data and tools from Sage Transparency which incorporates content from RAND Corporation, the National Academy for State Health Policy (NASHP), and other sources. It also offers guidance on rights and responsibilities as plan sponsor fiduciaries to determine fair prices for hospital services, market- and policy-based strategies, and ways to work individually and through coalitions to achieve fair pricing for hospital services.
While there isn’t a one-size-fits-all approach as available data and market conditions vary among regions and states, the methodology and action steps in the playbook to help plan sponsors determine and achieve a fair price include:
Identify breakeven costs – Uncover what hospitals need to charge commercial customers to break even considering all other incomes and expenses plus a reasonable margin.
Compare costs among peer hospital systems – Determine how hospital charges compare to other hospitals with similar services and quality.
Determine a fair market price – Use data from Sage Transparency to negotiate fees based on a reasonable markup of hospital costs.
The playbook also includes an overview of the Consolidated Appropriations Act and debunks many of the inaccuracies around hospital pricing with a comprehensive resource – “Myths and Facts: Revealing Hospital Price Transparency Truths.”
Enjoy.