From Capitol Hill, Govexec informs us that
Congress is looking to fund federal agencies at their current spending levels through mid-December, with momentum growing for a 10-week stopgap bill to avoid a government shutdown on Oct. 1.
Lawmakers must clear several hurdles before voting on a continuing resolution to kick off fiscal 2023, but they could act as soon as this week. Senate Majority Leader Chuck Schumer, D-N.Y., said last week he would work with Republicans to “avoid even a hint of a shutdown,” though several disagreements remain. Negotiations appear to have settled on a CR that would fund agencies through approximately Dec. 16, but lawmakers have yet to determine exactly which provisions will be added to it.
From the federal appointment front, STAT News reports
President Joe Biden on Monday appointed longtime biologist and former government scientist Renee Wegrzyn as the first director of the nascent Advanced Research Projects Agency for Health.
Biden’s announcement comes as ARPA-H advocates debate where the multibillion-dollar agency should be headquartered and which elusive disease areas should be prioritized. The president officially launched the agency in March with $1 billion in initial funding allotted by Congress, but the search for its inaugural director has taken months.
Wegrzyn, 45, currently works at Boston-based Ginkgo Bioworks, a company focused on biological engineering, but has prior experience in two government agencies Biden has said he hopes to emulate with ARPA-H — the Pentagon’s Defense Advanced Research Projects Agency and the Intelligence Advanced Research Projects Activity.
Good luck, Dr. Wegryzn.
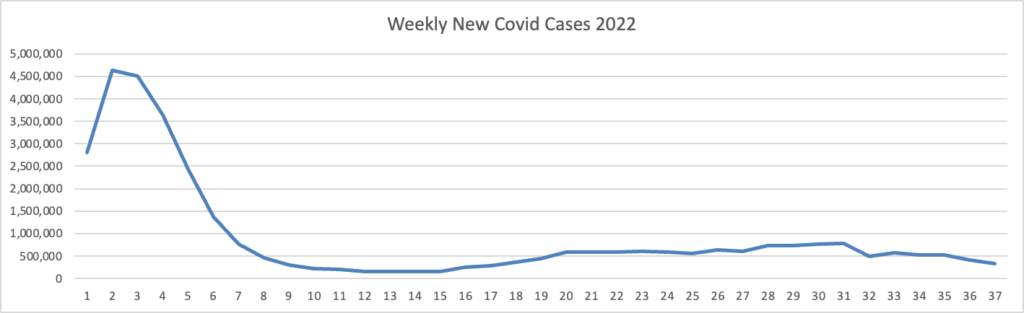
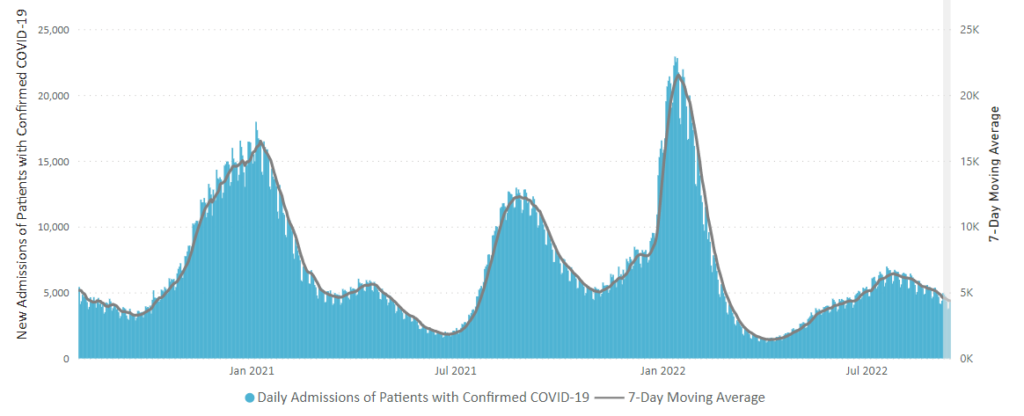
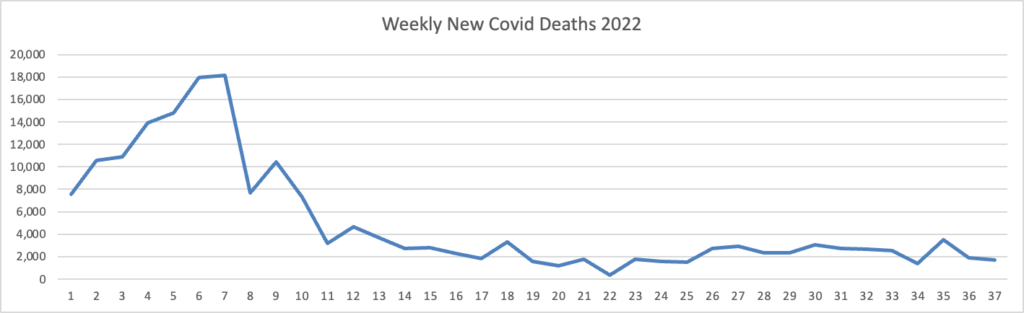
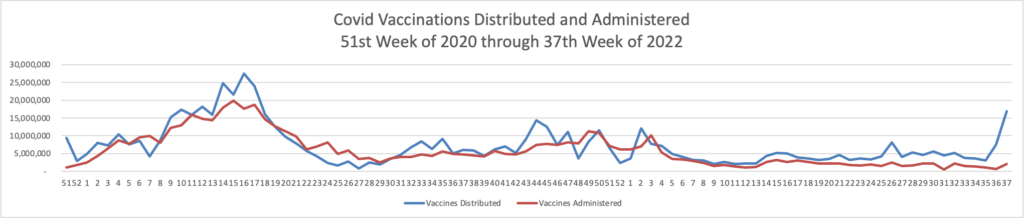
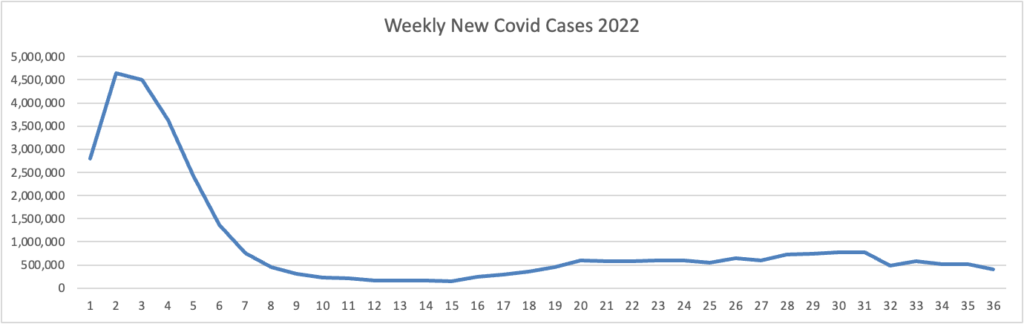
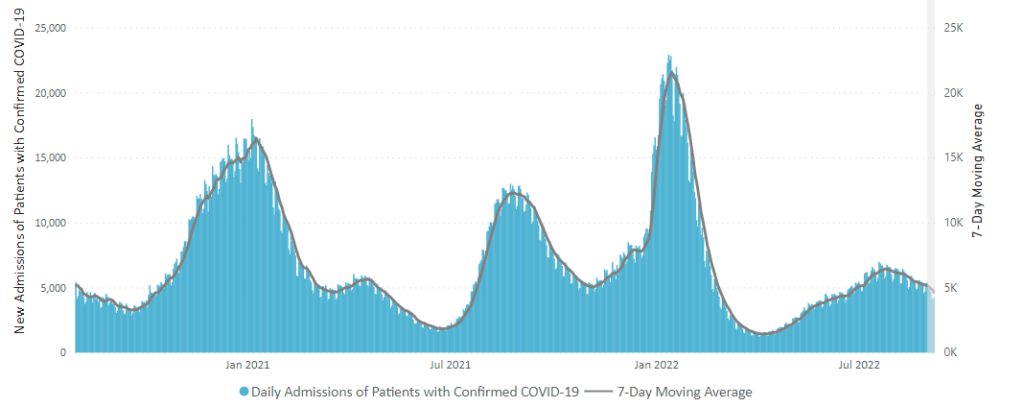
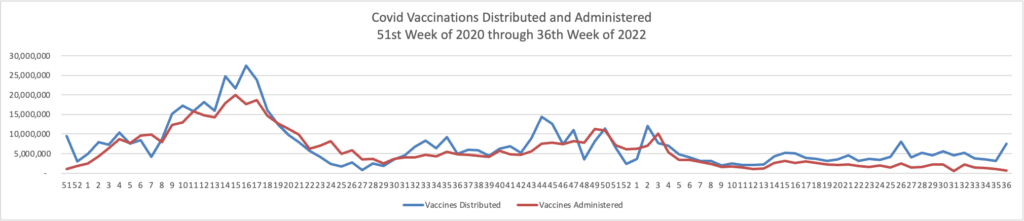
From the omicron and siblings front
The American Hospital Association tells us
Insured and uninsured Americans can receive the new bivalent Pfizer or Moderna COVID-19 booster and other COVID-19 vaccines at no cost as long as the federal government continues to purchase and distribute them, the Centers for Medicare & Medicaid Services announced today.
The Centers for Disease Control and Prevention this month recommended Pfizer’s updated COVID-19 vaccine booster for Americans aged 12 and older and Moderna’s updated COVID-19 vaccine booster for Americans aged 18 and older at least two months after completing a primary COVID-19 vaccine series or booster. Authorized by the Food and Drug Administration, the updated boosters are bivalent, meaning they help protect against the most recently circulating omicron variants as well as the original virus strain.
Americans can find local sites administering the new COVID-19 vaccine booster here. For more on provider requirements and payment, visit the CDC COVID-19 Vaccination Program and CMS toolkit.
The Wall Street Journal reports
Illness caused by Covid-19 shrank the U.S. labor force by around 500,000 people, a hit that is likely to continue if the virus continues to sicken workers at current rates, according to a new study released Monday.
Millions of people left the labor force—the number of people working or looking for work—during the pandemic for various reasons, including retirement, lack of child care and fear of Covid. The total size of the labor force reached 164.7 million people in August, exceeding the February 2020 prepandemic level for the first time. The labor force would have 500,000 more members if not for the people sickened by Covid, according to the study’s authors, economists Gopi Shah Goda of Stanford University and Evan J. Soltas, at the Massachusetts Institute of Technology.
“If we stay where we are with Covid infection rates going forward, we expect that 500,000-person loss to persist until either exposure goes down or severity goes down,” said Mr. Soltas. That assumes that some of those previously sickened eventually return to work.
The authors “provide the most credible evidence to date about labor-market impacts for a large set of workers,” said Aaron Sojourner, an economist at the W.E. Upjohn Institute for Employment Research, who wasn’t involved in the study.
From the U.S. healthcare business front —
Healthcare Dive relates
Escalating costs for labor, drugs, supplies and equipment are adding to the long-term pressures facing rural hospitals, raising the risk of more closures that could jeopardize patient access to care, the American Hospital Association warned in a new report.
Many hospitals were already in difficult financial positions before the COVID-19 pandemic began, due to challenges including low patient volume and reimbursement, geographic isolation, staffing shortages and aging infrastructure, the AHA said. From 2010 through 2021, 136 rural hospitals closed, according to data from the University of North Carolina’s Cecil G. Sheps Center for Health Services Research. In 2020, when the pandemic hit, a record 19 rural hospitals closed.
The public health emergency put additional pressure on margins and patient volumes. “While rural hospitals were partially buoyed by the Provider Relief Fund and other sources of COVID-19 assistance that limited closures in 2021, the financial outlook for many rural hospitals moving forward is precarious,” the AHA said.
Revcycle Intelligence reports
Private equity acquisition of physician practices in dermatology, gastroenterology, and ophthalmology was associated with increased healthcare spending and utilization, according to a study published in JAMA Health Forum. * * *
Following a private equity acquisition, physician practices saw consistent growth in spending during the next eight quarters. Acquired practices saw a mean increase of $71 in charges per claim or a 20.2 percent increase. In addition, practices saw an increase of $23 in the allowed amount per claim—an 11 percent increase.
Patient utilization of healthcare services grew as well after practices underwent acquisitions.
Across the eight post-acquisition quarters, the mean number of unique patients increased by 25.8 percent. This increase was mainly driven by more new patient visits, which rose by 37.9 percent. The number of encounters grew by 16.3 percent and the number of evaluation and management (E/M) visits increased by 37.1 percent.
The increase in patient visits may reflect changes in management and practice operations or overutilization of profitable services and low-value care, the study suggested. This could lead to higher healthcare spending without corresponding benefits.
Additionally, researchers said the growing number of visits was consistent with private equity firms’ common strategy to maximize revenue through a fee-for-service delivery system.
Ruh roh on both counts.
Healthcare Finance adds
Quality can go a long way in determining if a consumer is willing to pay more for their healthcare, as indicated by new survey responses published by revenue cycle company AKASA.
Out of more than 2,000 respondents, the survey found that 57% would pay more for a higher quality of care. Out of all categories in the survey, care quality was the only area in which a majority said they would be willing to pay more.
Forty-seven percent said they would pay more for the ability to work with the care team of their choice. Forty-one percent said they would pay more for the ability to work with hospitals of their choice, while the same percentage said they’d pony up more cash for better location proximity or convenience.
In public health news, the American Hospital Association celebrates the fact that
The United Network for Organ Sharing, which serves as the nation’s transplant system under contract with the federal government, Friday reported its millionth U.S. organ transplant. UNOS and the Organ Donation and Transplantation Alliance credited the organ donation and transplantation community, including transplant hospitals, with making the historic milestone possible. The first successful transplant took place at Peter Bent Brigham Hospital (now Brigham and Women’s Hospital) in Boston in 1954.
UNOS and the Alliance encourage the transplant community to join Living It Forward, a national initiative to commemorate the achievement and accelerate the path forward to the next million transplants. They also encourage members of the public to register as organ donors, noting that each donor can save up to eight lives and help up to 75 people through tissue donation. Over 100,000 people remain on the transplant waitlist.
From the Rx coverage front, BioPharma Dive tells us that
The Food and Drug Administration on Friday approved Bristol Myers Squibb’s psoriasis pill Sotyktu, the first medicine of its type and the last of three potential blockbuster drugs the company sought to bring to market this year.
Sotyktu will compete with biologic drugs like AbbVie’s Humira and Amgen’s Enbrel, but as a pill could be more attractive to patients who don’t want to inject themselves regularly. Importantly, Sotyktu’s labeling doesn’t require patients to first try biologic drugs, giving doctors an opportunity to prescribe it widely.
Approval came after Phase 3 testing in which the pill, also known as deucravacitinib, was tested against a placebo as well as another oral therapy, Amgen’s Otezla. In patients with moderate-to-severe plaque psoriasis, Sotyktu outperformed both on two commonly used measures for assessing skin clearing: PASI and sPGA.
“All in all, the overall efficacy and safety profile as a new first-in-class agent for plaque psoriasis bodes well for it becoming the standard of care,” said Samit Hirawat, Bristol Myers Squibb’s chief medical officer, setting a high bar for his company’s commercial expectations.
In wellness news, Healio informs us that
Widespread adoption of simple lifestyle changes, including switching to a well-known eating plan, could reduce risk for CV events and death for millions of adults with stage 1 hypertension, researchers reported.
“Millions of working-age people are walking around with elevated BP, which is symptomless but is also a leading preventable cause of disability and death,” Kendra D. Sims, PhD, MPH, a postdoctoral fellow at the University of California, San Francisco (UCSF) School of Medicine who presented the findings at the American Heart Association Hypertension Scientific Sessions, told Healio. “Our study found that 27,000 CVD events and 2,800 deaths could be prevented during the next 10 years if people with elevated BP follow through with recommended lifestyle changes. We would then save $1.6 billion in associated health care costs. The largest benefit comes from eating more fruits and vegetables and less salt, as outlined in the Dietary Approaches to Stop Hypertension (DASH) diet.