Thursday Miscellany

From Capitol Hill, STAT News reports
The Senate Judiciary Committee on Thursday passed legislation to prevent drug companies from gaming the patent system to delay competition from cheaper generics, but members in both parties said they still have concerns about the reforms.
It’s unclear when the bills might advance in either chamber.
The Congressional Research Service released an analysis of healthcare coverage spending in 2021.
Meanwhile, the Health Affairs Council on Healthcare Spending and Value updates us on the recommendations proposed in its 2018 Road Map for Action.
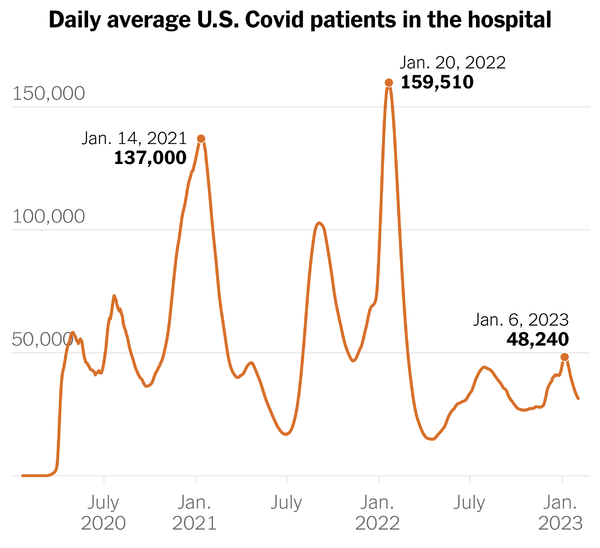
From the Omicron and siblings front —
The Department of Health and Human Services (HHS) announced that its Secretary Xavier Becerra had given the States 90 days advance notice of the end of the Covid public health emergency on May 11, 2023.
To help you and your communities in your preparations for the end of the COVID-19 PHE, I have attached a fact sheet to this letter that includes information on what will and will not be impacted by the end of the COVID-19 PHE.2 In the coming days, the Centers for Medicare & Medicaid Services (CMS) will also provide additional information, including about the waivers many states and health systems have adopted and how they will be impacted by the end of the COVID-19 PHE. I will share that resource with your team when available.
MedPage Today informs us,
Early treatment with a single dose of pegylated interferon lambda in a highly vaccinated population of COVID-19 outpatients decreased the risk for hospitalization and emergency department (ED) visits lasting more than 6 hours, the phase III TOGETHER trial found.
Among nearly 2,000 participants with acute COVID symptoms and a risk factor for severe illness, 2.7% of those who received pegylated interferon lambda within a week of symptoms required hospitalization or ED visits, as compared with 5.6% of those given placebo (relative risk [RR] 0.49, 95% Bayesian credible interval [CrI] 0.30-0.76), reported Gilmar Reis, MD, PhD, of McMaster University in Hamilton, Ontario, and colleagues.
Results were similar regardless of vaccination status (over 80% were vaccinated), and the treatment effect with the long-acting form of interferon lambda-1 was more pronounced in those who received the subcutaneous injection with 3 days of their symptoms.
From the miscellany department —
HHS released initial guidance for Medicare’s Prescription Drug Inflation Rebate Program created by last year’s Inflation Reduction Act.
Under the Medicare Prescription Drug Inflation Rebate Program, drug companies who raise prices faster than the rate of inflation will be required to pay rebates to the Medicare Trust Fund. Below is a timeline of key dates for implementing the Medicare Prescription Drug Inflation Rebate Program:
- October 1, 2022: Began the first 12-month period for which drug companies will be required to pay rebates to Medicare for raising prices that outpace inflation on certain Part D drugs.
- January 1, 2023: Began the first quarterly period for which drug companies will be required to pay rebates for raising prices that outpace inflation on certain Part B drugs.
- April 1, 2023: People with Traditional Medicare and Medicare Advantage may pay a lower coinsurance for certain Part B drugs with price increases higher than inflation.
- 2025: CMS intends to send the first invoices to drug companies for the rebates.
The law has a circular aspect because the government needs a much lower general inflation index to get the full bang for the buck from this program. The notice also poses issues for public input.
The International Foundation of Employee Benefit Plans tells us,
The International Foundation has been tracking fertility and family-forming benefits over the past seven years. According to Employee Benefits Survey: 2022 Results, 40% of U.S. organizations currently offer fertility benefits (an increase from 30% in 2020).
Overall:
- 28% cover fertility medications (8% covered in 2016, 14% in 2018, 24% in 2020)
- 30% cover in vitro fertilization (IVF) treatments (13% in 2016, 17% in 2018, 24% in 2020)
- 16% cover genetic testing to determine infertility issues (11% in 2018, 12% in 2020)
- 17% cover non-IVF fertility treatments (6% in 2016, 11% in 2018, 11% in 2020).
In 2016, only 2% of organizations covered egg harvesting/freezing services. That jumped to 6% in 2018, 10% in 2020 and even higher in 2022, with 14% reporting that they cover the benefit.
Healthcare Dives points out, “National telehealth utilization increased 1.9% month-over-month among the privately insured population in November 2022, following one month of decline, according to a new analysis from Fair Health’s monthly tracker.” The bump is attributable to the tripledemic.
Fierce Healthcare relates, “UnitedHealthcare is rolling out a new wearables-based rewards program for members and their spouses. In UnitedHealthcare Rewards, eligible members can earn up to $1,000 per year by using wearable devices to complete health goals and activities, the insurance giant announced Wednesday.”
Health Payer Intelligence notes that “High deductible health plan (HDHP) enrollment hit a record high in 2021, with nearly six out of ten employer-sponsored health plan members enrolled in a high deductible health plan, according to a ValuePenguin survey.”
Benefits consultant Tammy Flanagan writing in Govexec, explains how federal employees can get the full advantage out of the Thrift Savings Plan, which is part of the Federal Employees Retirement System.