Midweek Update

Happy St. Patricks Day to all.
Congress in a recent appropriations law sought a report from the National Academy for Public Administration on the Trump Administration’s proposal to break up the Office of Personnel Management. The Federal Times reports that “A better federal workforce policy will require the Office of Personnel Management to become a more independent and authoritative agency, rather than being broken apart into other departments, a long-awaited National Academy for Public Administration study determined March 17. ”
“We strongly recommend that a central personnel agency continue to exist, and that organization is an independent, enterprise-wide human capital agency and a steward of the merit system principles,” said Terry Gerton, president and CEO of NAPA, said at a press briefing on the report. “But that organization, OPM as we know it today, really needs to build its staff capacity, encourage innovation and adopt a more data-driven, accountable and forward-looking capital management approach.”
In other federal agency news, the Federal News Network informs us that
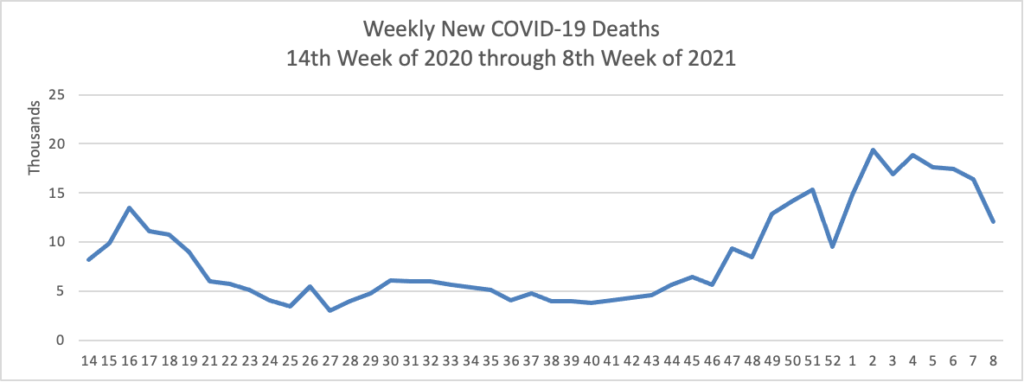
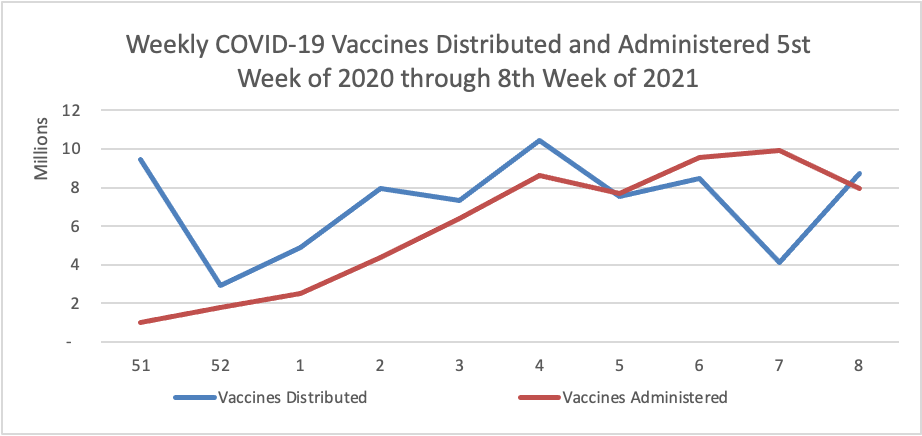
The IRS is moving this year’s April 15 tax filing season deadline back to May 17, citing ongoing challenges from the COVID-19 pandemic. IRS Commissioner Chuck Rettig said in a statement Wednesday that the new deadline would give the public more time to take stock of their finances, while also giving agency employees more time to implement new responsibilities under the American Rescue Plan.
The Department of Health and Human Services announced today that the agency
will invest $10 billion from the American Rescue Plan to ramp up screening testing to help schools reopen, $2.25 billion to scale up testing in underserved populations, and provide new guidance on asymptomatic screening testing in schools, workplaces, and congregate settings. These measures are part of President Biden’s strategy to increase COVID-19 testing nationwide as vaccinations increase.
“COVID-19 testing is critical to saving lives and restoring economic activity,” said HHS Acting Secretary Norris Cochran. “As part of the Biden Administration’s National Strategy, HHS will continue to expand our capacity to get testing to the individuals and the places that need it most, so we can prevent transmission of the virus and defeat the pandemic.”
The U.S. Preventive Services Task Force announced yesterday a proposed B grade recommendation “on screening for prediabetes and type 2 diabetes. The Task Force recommends screening adults between ages 35 to 70 years old who are overweight or obese for prediabetes and diabetes.” The current minimum age for this screening recommendation is 40 years old. NBC News reports that
We know the rates of prediabetes and diabetes are increasing in people who are younger,” said Dr. Chien-Wen Tseng, a task force member and a professor of family medicine at the University of Hawaii’s John A. Burns School of Medicine. “Our main reason for dropping the age is to match the screening with where the problem is: If diabetes and prediabetes are occurring at a younger age, then we should be screening at a younger age.”
The Affordable Care Act requires health plans to cover USPSTF A and B recommended preventive services without member cost sharing when an eligible member receives the service in-network. If the USPSTF finalizes this recommendation later in 2021, then the ACA health plan coverage mandate for this screening would expand to people in the age 35 to 40 age group in 2023. Here is a link to background on the USPSTF’s sixteen members.
In other health industry news —
Healthcare Dive reports that
Amazon is expanding its virtual care pilot program, Amazon Care, to employees and outside companies nationwide beginning this summer in a major evolution of its telehealth initiative, as the COVID-19 pandemic continues to drive unprecedented demand for virtual care.
Amazon will also offer its on-demand primary care service to other Washington state-based companies and plans to expand its in-person service to Washington, D.C., Baltimore and other cities in the following months, the e-commerce behemoth announced Wednesday.
Employee Benefit News offers a Chief Financial Offer’s advice on health savings account funding.