Weekend update

The House of Representatives and the Senate will be engaged in Committee business and floor voting this coming week. The Senate’s Executive Calendar states that
Ordered, That at 5 p.m. on Monday, June 14, 2021, the Senate proceed to executive session to resume consideration of the nomination of Ketanji Brown Jackson, of the District of Columbia, to be United States Circuit Judge for the District of Columbia Circuit.
Ordered further, That at 5:30 p.m., all post-cloture time expire.
Ordered further, That following disposition of the Jackson nomination the cloture motions with respect to the nominations of Lina M. Khan, of New York, to be a Federal Trade Commissioner for the unexpired term of seven years from September 26, 2017 and Kiran Arjandas Ahuja, of Massachusetts, to be Director of the Office of Personnel Management for a term of four years, ripen.
Ordered further, That with respect to the motions to invoke cloture on Kahn and Ahuja nominations, the mandatory quorum calls required under Rule XXII be waived.
Ordered further, That if any of the nominations are confirmed, the motions to reconsider be considered made and laid upon the table and the President be immediately notified of the Senate’s actions. (Jun. 10, 2021.)
The FEHBlog will continue to keep an eye on Ms. Ahuja’s nomination.
Here’s a link to the Blue Cross FEP website for its COVID-19 vaccination incentive. FedSmith has a complete report on his experience with the new program.
Fierce Healthcare reports that
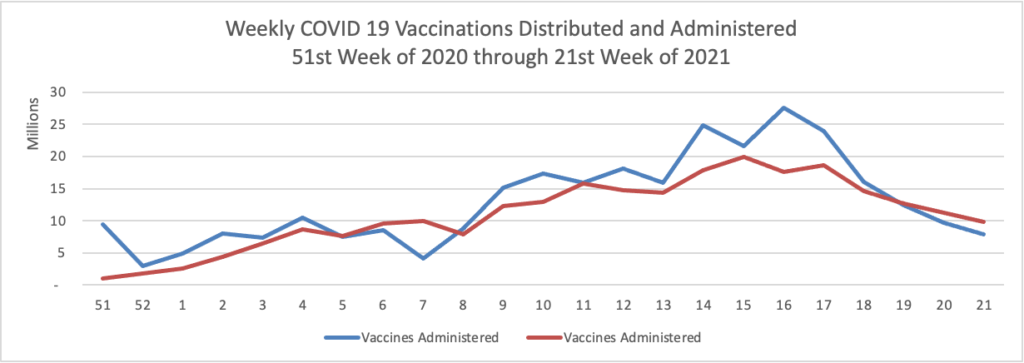
More than 96% of U.S. physicians have been fully vaccinated for COVID-19, with no significant difference in vaccination rates across regions, according to a new survey from the American Medical Association (AMA).
Of the physicians who are not yet vaccinated, an additional 45% do plan to get vaccinated, the AMA survey data (PDF) shows. The most common reason for not receiving the vaccine was that it was too new and has unknown long-term effects, according to physician responses.
The national AMA survey polled 300 physicians, including primary care doctors and specialists, between June 3-8. It’s the first survey to specifically collect data on practicing physicians’ COVID-19 vaccination rates, according to the AMA.
The survey results show an increase of more than 20% for physicians who have been fully vaccinated for COVID-19 compared to a May 2021 Medscape poll, AMA said.
“Practicing physicians across the country are leading by example, with an amazing uptake of the COVID-19 vaccines,” said AMA President Susan R. Bailey, M.D. in a statement.
In other encouraging news, the Wall Street Journal reports
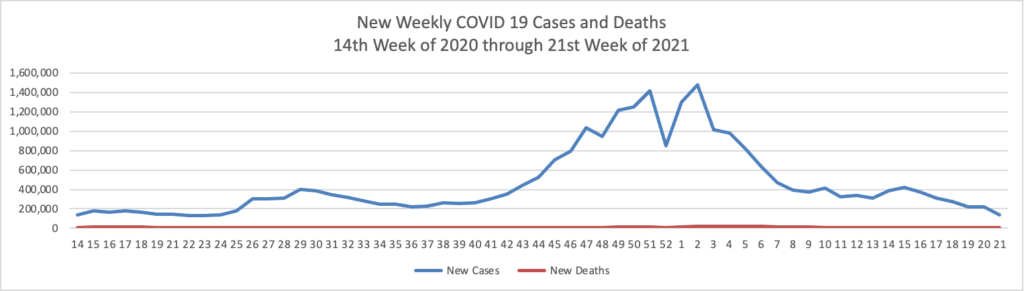
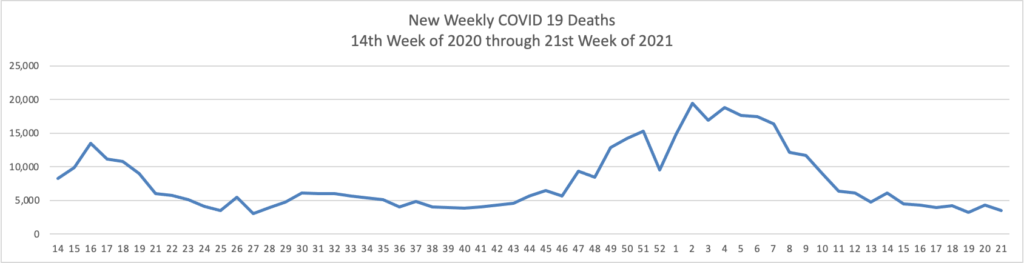
The proportion of Covid-19 laboratory tests that are coming back positive is at the lowest recorded point since the pandemic took hold in the U.S., a sign of progress as the country moves ahead with reopening. * * *
The proportion of tests coming back positive has been consistently falling since April this year and is now at the lowest point since March 2020, the furthest back the Johns Hopkins data are available.
The low positivity rate [2%] is a signal that the drop in infections is really due to less disease in the country rather than because the U.S. is testing less for the virus, according to epidemiologists. It is another indication of how the U.S. is gaining ground against the Covid-19 pandemic, along with declining case counts, hospitalizations and deaths.
“It’s a true reflection of a decrease in overall circulation in the U.S.,” said Anne Rimoin, an infectious-disease epidemiologist at the UCLA Fielding School of Public Health. “But we still do have pockets where we’re seeing transmission of the virus, and we need to be careful.”
NBC News adds that “There are only three Covid-19 patients at Sandra Atlas Bass Heart Hospital at North Shore University Hospital, on Long Island, New York — a far cry from when the hospital, which is part of Northwell Health, had as many as 600 patients during the peak of the pandemic. All three patients, who are in the intensive care unit, have one thing in common, said Dr. Hugh Cassiere, director of the hospital’s critical care services: They’re unvaccinated. The trend appears to be occurring at hospitals nationwide. “I haven’t had anyone that’s been fully vaccinated become critically ill,” said Dr. Josh Denson, a pulmonary medicine and critical care physician at Tulane University Medical Center in New Orleans.”
As the FEHBlog mentioned in Friday’s post, the federal government’s semi-annual regulatory agenda was posted on June 11. Over the course of this week, the FEHBlog will highlight the FEHB rule makings. Of note
OPM proposes to amend the Federal Employees Health Benefits Acquisition Regulation (FEHBAR) to update reporting requirements for health insurance carriers providing benefits through the Federal Employees Health Benefits (FEHB) Program. In the course of business, FEHB carriers collect pharmacy, enrollment, provider, person-specific medical and pharmacy claims, or in the case of managed care plans, encounter data related to enrollees and family members in order to provide healthcare coverage to those individuals. Under this proposed regulation, FEHB carriers would be required to submit this information to OPM, related to all benefits and services provided under the Program, on no less than an annual basis. This rule clarifies the requirements for FEHB carriers to furnish such reasonable reports, pursuant to 5 U.S.C. 8910, to better enable OPM, as a health oversight agency, to obtain the information necessary for proper administration of the FEHB Program.
View Rule (reginfo.gov) OPM first proposed to create an FEHB identifiable claims data warehouse in 2010. In 2015 OPM reported a massive data breach. Logically OPM should have backed off the idea of an FEHB identifiable claims data warehouse because that would be such an attractive target for hackers. It didn’t; OPM proposed an FEHB rule to create the identifiable claims data warehouse in September, 2019, and the rule making died at the Office of Management and Budget review stage three months later. OPM now has decided to take another bite at the apple; this time with an acquisition regulation.
OPM also has its own No Surprises Act rule making which includes the independent dispute resolution processes.
This interim final rule with comment would implement additional protections against surprise medical bills under the No Surprises Act, including provisions related to the independent dispute resolution processes.
New Section 8902(p) of the FEHB Act requires OPM to implement certain No Surprises Act provisions in the FEHB carrier contracts and to extend corollary obligations on healthcare providers by rule making. OPM states that this rule has a statutory deadline of October 1, 2021. HHS also is scheduled to release Part II of its No Surprises Act interim final rule making by the same date. The FEHBlog noted last week that Part I of that HHS rule making which has a statutory deadline of July 1, 2021, is pending OMB review.