Thursday Miscellany
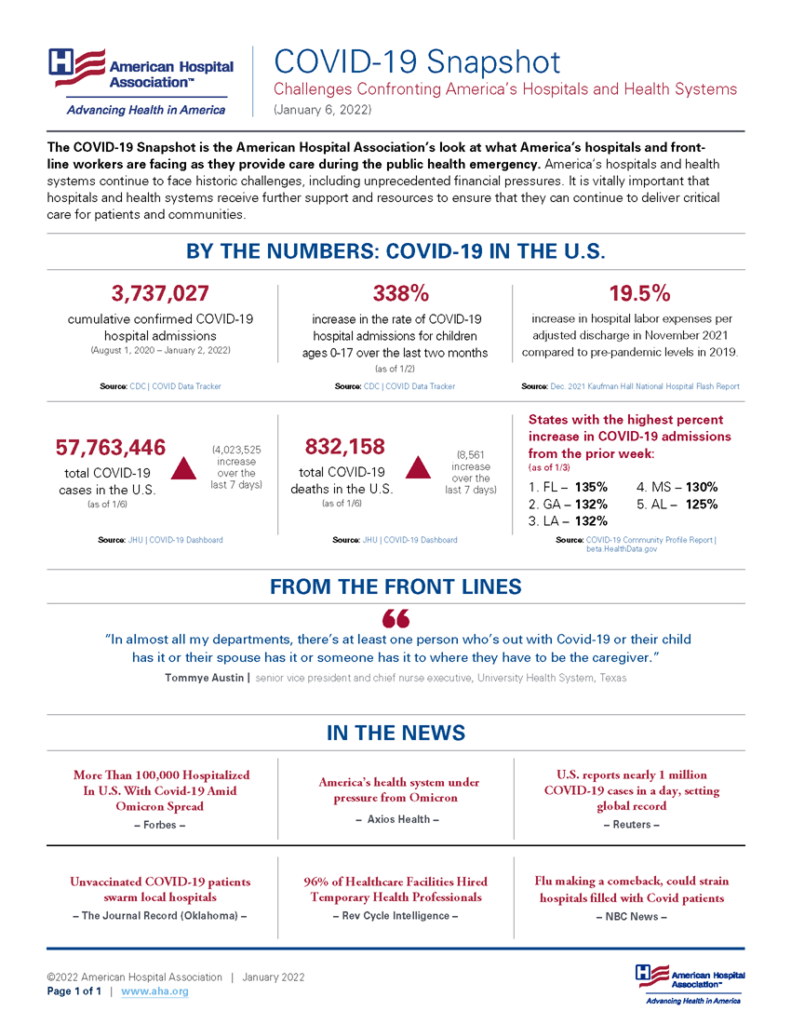
From the Omicron front, the American Hospital Association offers its January 2022 monthly COVID snapshot. “The COVID-19 Snapshot is the American Hospital Association’s look at what America’s hospitals and frontline workers are facing as they provide care during the public health emergency.”
From the COVID testing front, the FEHBlog commends this 20 minute long Wall Street Journal podcast on why at home COVID tests are so hard to find.
In that regard STAT News offers a report on a
new study raises significant doubts about whether at-home rapid antigen tests can detect the Omicron variant before infected people can transmit the virus to others.
The study looks at 30 people from settings including Broadway theaters and offices in New York and San Francisco where some workers were not only being tested daily but were, because of rules at their workplaces, receiving both the antigen tests and a daily test that used the polymerase chain reaction, or PCR, which is believed to be more reliable.
On days 0 and 1 following a positive PCR test, all of the antigen tests used produced false-negative results, even though in 28 of the 30 cases, levels of virus detected by the PCR test were high enough to infect other people. In four cases, researchers were able to confirm that infected people transmitted the virus to others during the period before they had a positive result on the rapid antigen test.
This study suggests to the FEHBlog that one rapid at home tests are still useful to help decide whether to end an isolation or quartine period at five days or once available whether or not a COVID pill should be taken. However, one use of a rapid at home test may not be so reliable to help decide whether or not to enter a gathering. In fairness to Abott and Quickvue, both manufacturers include two tests in each kit so that the test can be repeated a couple days later. If you are using these at home tests follow the manufacturers’ instructions.
From the COVID vaccine mandate front, the U.S. Court of Appeals for the Sixth Circuit on January 5 upheld by a 2-1 vote a regional stay on the Biden Administration’s government contractor mandate. As you know the U.S. Court of Appeals for the 11th Circuit is considering a nationwide stay on that mandate after deciding the stay may remain in place pending the Court’s final decision on that matter.
On Friday morning the U.S. Supreme Court will consider whether or not to maintain stays on the Biden Administation’s healthcare worker vaccination mandate and its OHSA ETS vaccinate or test program for employers with 100 or more employees. The Supreme Court allows the public to listen to the oral argument or read the transcript. The proceedings will begin at 10 am ET. The FEHBlog expects a decision from the Court in this accelerated proceeding next month.
On the private sector initiated vaccine front, the Society for Human Resource Management (SHRM) adds that
Employers are on safer legal footing terminating employees for violating mandatory vaccination policies than imposing lesser punishments, legal experts advise. They say employers should not opt, for example, to withhold pay raises, make only vaccinated workers eligible to apply for internal positions or promote only vaccinated employees.
Many lower courts have upheld employers’ mandatory vaccination policies, which, unlike the Occupational Safety and Health Administration’s emergency temporary standard, aren’t yet before the Supreme Court, though the court may address them in passing.
On the human resources front —
- SHRM offers 2022 guidance and reminders for benefit and compensation managers.
- Govexec digs into the details of the President’s recent executive order adjusting federal employee compensation for 2022.
From the healthcare business front —
Healthcare Dive tells us that
Value-based primary care provider Vera Whole Health has announced plans to acquire health data and navigation company Castlight in a deal valued at approximately $370 million.
The transaction, which will bring a value-based care model to the employer healthcare market by integrating Castlight’stechnology with Vera’s clinical network and medical workers, is structured as an all-cash tender offer under which Vera will acquire all of Castlight’s outstanding shares.
Vera’s majority equity holder, Clayton, Dubilier & Rice, has committed to invest up to $338 million in the combination, while major health insurer Anthem (a long-time Castlight customer) has also pledged to make an investment, the size of which has yet to be disclosed.
Fierce Healthcare reports that
Magellan Healthcare has partnered with NeuroFlow to launch a digital emotional well-being program accessible to all Magellan members.
The program went live at the start of the new year and aims to be a self-help tool, enabling members to take charge of their own mental health and build confidence and stress-management skills. NeuroFlow supplies the technology via a member-facing platform, which can be accessed either via an iOS or Android app or web browser.
Members who use the platform can access exercises that are meant to keep them engaged and can receive recommendations for resources based on their needs. Activities include evidence-based videos, behavioral trackers and digital cognitive behavioral therapy programs developed by Magellan. The program can also refer members to a care manager or therapist.







