Friday Stats and More
Based on the Centers for Disease Control’s Covid Data Tracker and using Thursday as the first day of the week, here are the FEHBlog’s weekly charts of new Covid cases and deaths from the 27th week of 2021, a low points of cases and death, and the 14th week of 2022, another lull but not quite as low.
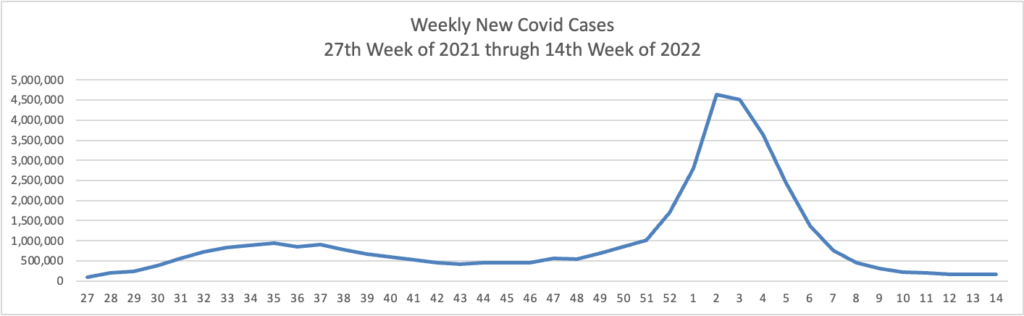
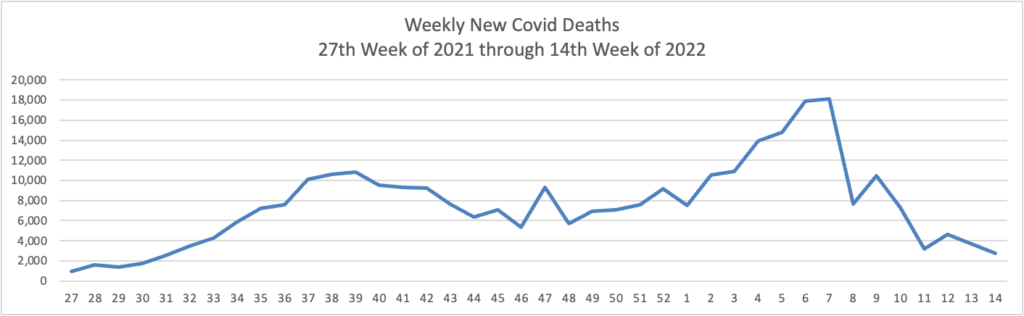
The CDC’s Weekly Review of its COVID statistics issued today notes “The current 7-day daily average for March 30–April 5, 2022, was 1,406. This is a 10.3% decrease from the prior 7-day average (1,567) from March 23–29, 2022.”
Here’s the FEHBlog’s weekly chart of Covid vaccinations administered and distributed since the beginning of the Covid vaccination era through this week, again using Thursday as the first day of the week.
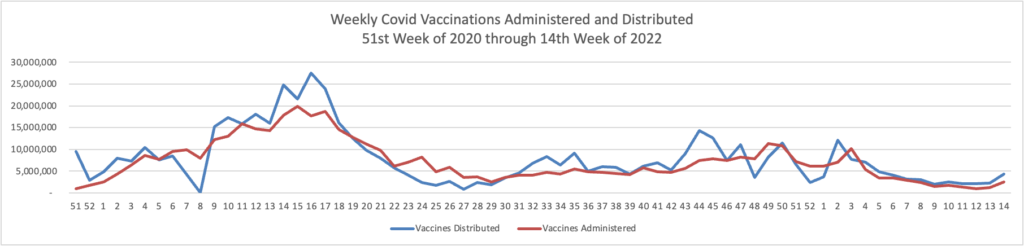
The administration of Covid vaccines popped us this week. Over 75% of the U.S. population aged 18 and older are fully vaccinated. Nearly half (48.6%) of that cadre is boostered. The Weekly Review’s commentary discusses the importance of vaccinating children.
COVID-19 vaccines have undergone—and continue to undergo—the most intensive safety monitoring in U.S. history, and adverse events are rare. Vaccinating children is the single best way to protect them from severe illness associated with COVID-19.
From Capitol Hill, the Wall Street Journal reports
Senators had also hoped to move forward on the coronavirus vaccines and treatments package, but progress quickly bogged down over Republican efforts to amend the bill to extend a pandemic-era immigration policy called Title 42—which allows Border Patrol agents to quickly turn away migrants at the southern border—with some Democrats siding with the GOP. Senators said they ran out of time, and the break could help end the logjam, even if it means the aid will need to wait at least several weeks.
“We’ll see where the discussions go, but my assumption is during the course of the break they’ll be some conversations between people who are interested in advancing it and see if we can make any headway on coming up with a process,” said Sen. John Thune (R., S.D.) on the Covid aid.
“I don’t think we’re leaving anything hanging up in the air that we’re not going to be able to continue to work with afterwards,” said Sen. Angus King (I., Maine), who caucuses with Democrats.
Congress is on State / District work periods for the next two weeks.
From the Rx coverage front, Health Affairs offers a fascinating article leading with an HHS Inspector General report on biosimilar drug use in the Medicare Part D program. The article blossoms into a broader look at biosimilar use in America. For example,
Last fall, two academics from the USC Schaeffer Center for Health Policy & Economics and the University of Chicago Harris School of Public Policy analyzed the available biosimiliars and found these were, on average, 30% less expensive than the underlying brand-name biologics. This represented a savings of about $665 off the average price.
Perhaps the biggest boost, though, will occur when biosimilar versions of Humira begin entering the U.S. market next year. This is expected to kick-start a wave of increased biosimilar usage between now and 2027, by which time the worldwide market should roughly double to $20 billion, according to Bernstein analyst Ronny Gal.
“The savings we identified with increased biosimilar use, while modest, could be significant once biosimiliar versions of Humira come on the market,” said [Melissa] Baker [from the HHS Office of Inspector Genera]. “Part D spending for Humira is in the billions of dollars.” The HHS OIG report noted that Humira and Enbrel accounted for more than $5 billion in Part D spending and nearly half of Part D spending on biologics in 2019.
From the Aduhelm front, Fierce Healthcare tells us
Leaders of the Food and Drug Administration (FDA) and the Centers for Medicare & Medicaid Services (CMS) sought to present a united front a day after CMS approved narrow Medicare coverage of the Alzheimer’s disease drug Aduhelm.
FDA Commissioner Robert Califf, M.D., and CMS Administrator Chiquita Brooks-LaSure issued a joint statement Friday to address criticism of CMS’ decision that Medicare only cover Aduhelm and similar products for beneficiaries in a qualifying clinical trial. Critics have charged CMS is trying to undermine the FDA’s approval decisions as the agency cleared the drug last year via accelerated approval.
“The work of both of our agencies is critical to ensure that medical products are available to people across the country,” the agency leaders said in a statement.
From the mental healthcare front, Health Payer Intelligence informs us
CVS Health and its payer arm, Aetna, aimed to make strides in the healthcare industry in 2021 by delivering affordable healthcare services to members, increasing access to virtual and mental healthcare, and implementing initiatives to advance health equity, according to the payer’s 2021 Environmental, Social, and Governance (ESG) report. The report reflects data from January 1 to December 31, 2021.
Well done.
Finally, the FEHBlog ran across this helpful Kaiser Family Foundation preventive services tracker website.
The Affordable Care Act (ACA) requires new private health insurance plans to cover many recommended preventive services without any patient cost-sharing. For adults, the required services are recommended by the U.S. Preventive Services Task Force (USPSTF), the Advisory Committee on Immunization Practices (ACIP), and the Health Resources and Services Administration (HRSA) based on recommendations issued by the Institute of Medicine Committee on Women’s Clinical Preventive Services. As new recommendations are issued or updated, coverage must commence in the next plan year that begins on or after exactly one year from the recommendation’s issue date.
This tracker presents up-to-date information on the adult preventive services nongrandfathered private plans must cover, by condition, including a summary of the recommendation, the target population, the effective date of coverage, and related federal coverage clarifications.
For more information, see the fact sheet Preventive Services Covered by Private Health Plans under the Affordable Care Act.
This tracker also applies to FEHB plans. Thanks, KFF.







