Friday Stats and More
Note — Unfortunately, Thursday’s post did not arrive on the E&S website until 9 am ET today, so it did not go out to subscribers this morning. Lo siento. Here is a link to yesterday’s post.
Onto today’s post —
Based on the CDC’s Covid Data Tracker and using Thursday as the first day of the week, here is the FEHBlog’s latest weekly chart of new Covid cases:
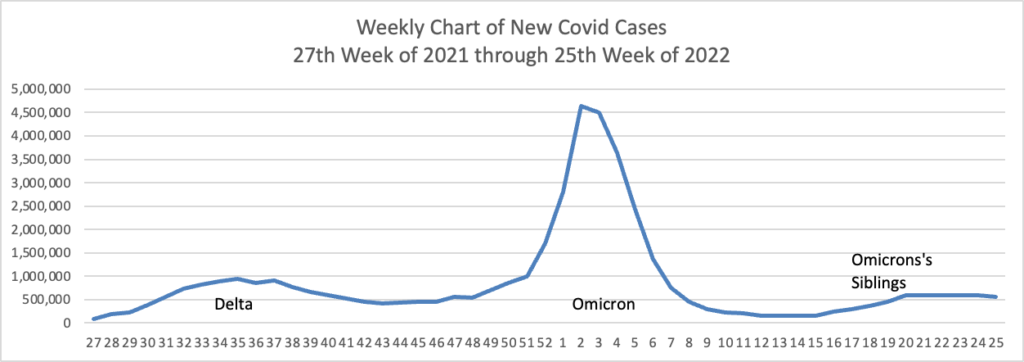
The CDC’s weekly review of its Covid statistics states “As of June 22, 2022, the current 7-day moving average of daily new cases (97,430) decreased 5.6% compared with the previous 7-day moving average (103,175).”
Here’s the CDC’s weekly chart of new Covid hospital admissions:
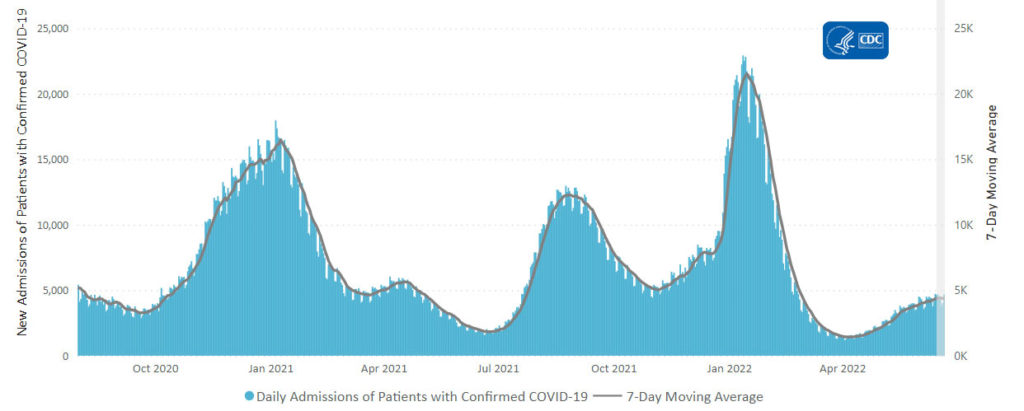
The CDC’s weekly review states “The current 7-day daily average for June 15–21, 2022, was 4,375. This is a 1.0% increase from the prior 7-day average (4,329) from June 8–14, 2022.”
Here is the FEHBlog’s latest weekly chart of new Covid deaths:
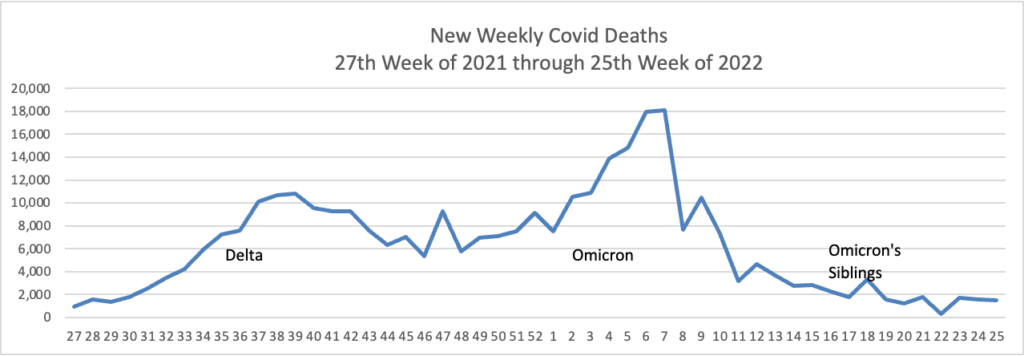
The CDC’s weekly review states “The current 7-day moving average of new deaths (255) has decreased 10.4% compared with the previous 7-day moving average (285).”
The CDC’s weekly review also reports
As of June 23, 2022, there are 391 (12.1%) counties, districts, or territories with a high COVID-19 Community Level, 996 (30.9%) counties with a medium Community Level, and 1,830 (56.8%) counties with a low Community Level. This represents an increase (+1.9 percentage points) in the number of high-level counties, a slight increase (+1.6 percentage points) in the number of medium-level counties, and a corresponding decrease (−3.6 percentage points) in the number of low-level counties. 51 jurisdictions had high- or medium-level counties this week. Rhode Island is the only jurisdiction to have all counties at low Community Level.
To check your COVID-19 Community Level, visit COVID Data Tracker. To learn which prevention measures are recommended based on your COVID-19 Community Level, visit COVID-19 Community Level and COVID-19 Prevention.
The weekly statistics generally are stable and moving in the right direction.
The American Hospital Association adds
The Centers for Disease Control and Prevention last night endorsed Moderna’s COVID-19 vaccine for children aged 6-17, as its advisory committee recommended, creating an alternative to Pfizer’s COVID-19 vaccine for this age group. The Food and Drug Administration authorized the Moderna vaccine for children and adolescents last week.
Before ACA FAQ 50 issued October 4, 2021, the period for covering COVID vaccines with no cost sharing began 15 days after the CDC’s action. The FEHBlog, who is not errorless, thought that FAQ 50 eliminated the 15 day waiting period, but upon further review, FAQ 50 requires immediate no cost sharing coverage of Covid vaccines filing the FDA’s approval, usually an emergency use authorization. The FEHBlog doesn’t think this makes any practical difference because the Covid vaccines aren’t distributed without CDC approval.
From the Capitol Hill, the American Hospital Association provide us with this encouraging news:
The House of Representative today voted 234-193 to pass and send to the President for his signature bipartisan legislation to help reduce gun violence in communities. Approved by the Senate last night, the AHA-supported package includes behavioral health provisions, including funding for school safety resources, school-based supportive services and expanded access to telehealth for mental and behavioral health services.
From the Supreme Court, the Court decided today that the right to an abortion is a matter controlled by state law, not the U.S. Constitution. The Wall Street Journal sums it up as follows “In upholding a Mississippi law banning the procedure after 15 weeks of pregnancy, the court’s conservative majority said the Roe decision was egregiously wrong in recognizing a constitutional right to abortion.” In response
- The Biden Administration created a reproductive rights website.
Reproductive health care, including access to birth control and safe and legal abortion care, is an essential part of your health and well-being. While Roe v. Wade was overturned, abortion remains legal in many states, and other reproductive health care services remain protected by law. The U.S. Department of Health and Human Services (HHS) is committed to providing you with accurate and up-to-date information about access to and coverage of reproductive health care and resources. Our goal is to make sure you have appropriate information and support.
- Health Payer Intelligence discusses health insurer reaction to the decision. “Payers and healthcare leaders are responding to the Supreme Court’s decision to overturn Roe v Wade, the case which protected abortion rights at the federal level, and while the repercussions remain uncertain many healthcare leaders are voicing their commitment to helping women navigate the impacts.”
- The Wall Street Journal discusses employer reaction to the decision. “Businesses with health plans covering abortion now are weighing whether and how to pay for employees to travel to a state where the procedure is legal.”
From the OPM front
- Federal News Network reports on OPM Director Karen Ahuja’s press conference held yesterday, the first anniversary of her swearing in as OPM Director.
- FedWeek tells us that “OPM has said it is working to improve features for federal employees and annuitants to compare FEHB plans, although it does not project having those improvements in place until late next year—potentially in time for that year’s open season for selecting coverage in 2024.”
From the nicotine front, the Wall Street Journal reports
A federal appeals court on Friday granted Juul Labs Inc. a temporary stay of the Food and Drug Administration’s order for the vaping company to pull its e-cigarettes off the U.S. market.
A panel of judges from the U.S. Court of Appeals for the D.C. Circuit on Friday afternoon granted Juul’s request to delay the FDA’s ban, according to court documents. The temporary stay gives the court time to hear arguments and wasn’t a ruling on the merits of the case, the judges wrote.
Finally, HR Dive brings us a roundup of happenings at this week’s Society for Human Resource Management conference.








