Tuesday Tidbits

The FEHBlog is back inside the Capital Beltway this week so let’s kick it off with a link to the President’s remarks at his signing of the budget reconciliation act (H.R. 5376) into law.
Because the new law’s drug pricing changes will impact the FEHB Program most, here is a link to STAT New’s article unpacking those provisions.
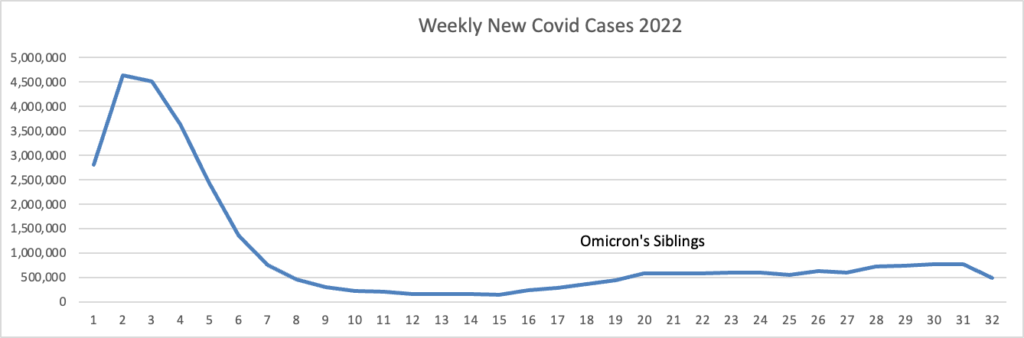
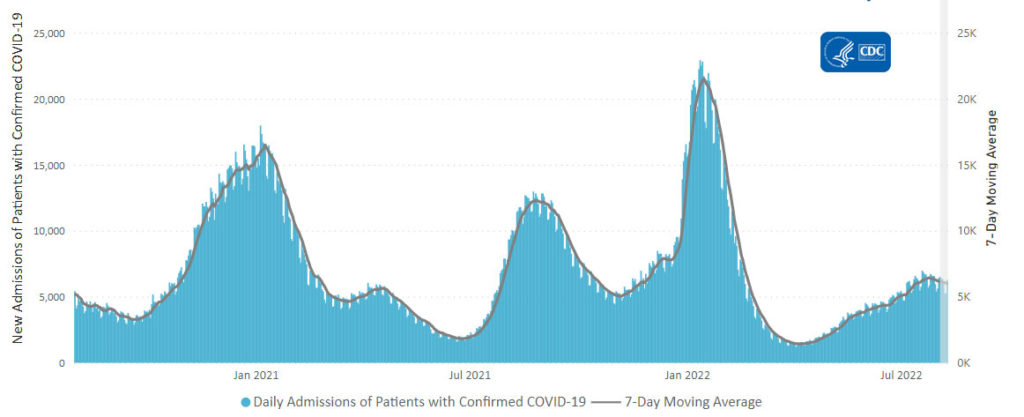
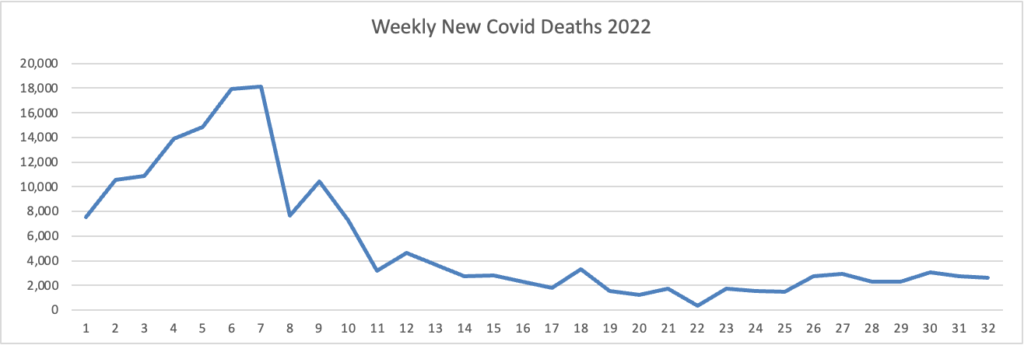
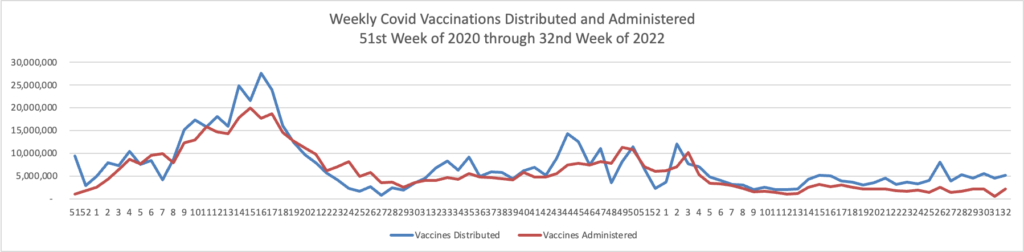
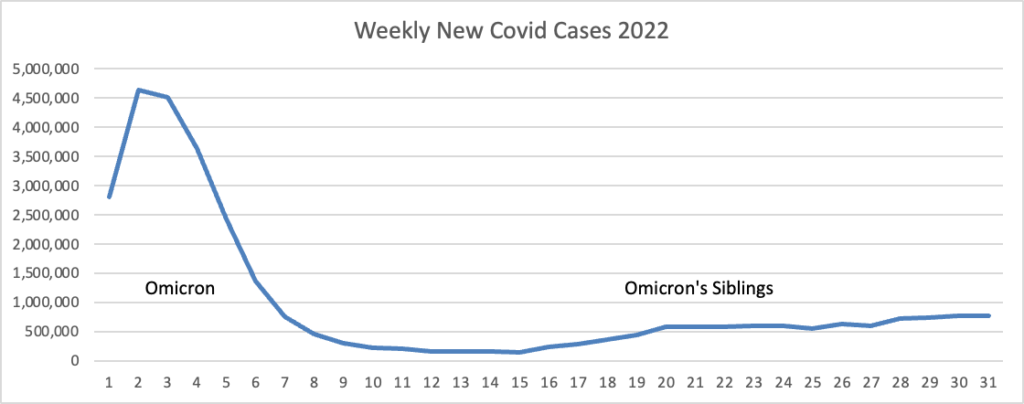
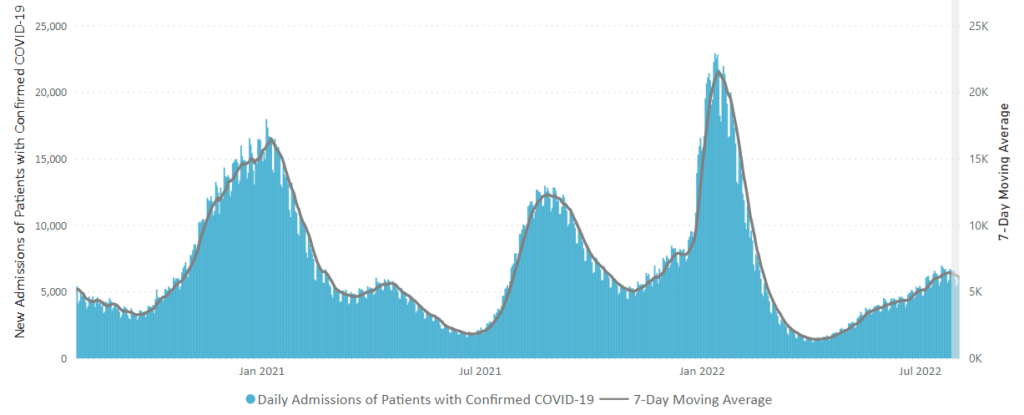
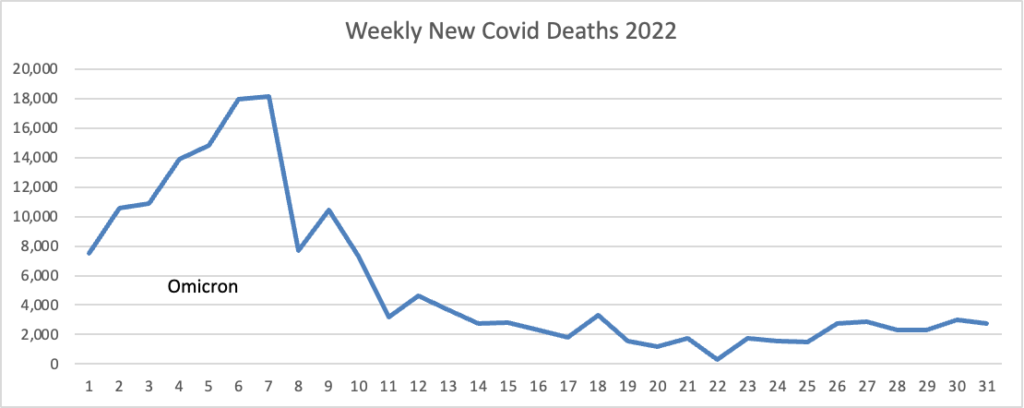
From the omicron and siblings front, Federal News Network reports that the Safer Federal Workforce Task Force is following the CDC’s lead by easing restrictions to stop the Covid spread.
From the monkeypox front, check out Fierce Healthcare’s monkeypox news tracker.
MedPage Today offers a fascinating article in which a Johns Hopkins epidemiologist assesses the current case of polio paralysis in New York through a historical lens.
Polio is a fecal-oral pathogen and the Sabin vaccine, as an oral vaccine, more naturally mimicked the natural route of infection and therefore triggers different and more potent immune responses than the injectable Salk vaccine. Also, because it is a “live” vaccine, the vaccine virus is shed and can passively immunize close contacts of vaccinated individuals. For these reasons, the Sabin vaccine eventually came to be a more dominant and preferred vaccine than the [original, injected] Salk version.
However, as polio receded as a public health threat, rare complications elicited by the Sabin vaccine became intolerable in many Western countries. The “live” nature of Sabin’s vaccine, in rare instances, could lead to vaccine-associated paralytic polio. In even rarer circumstances, the virus could mutate or recombine with other viruses to become a circulating vaccine-derived poliovirus (cVDPV) that could be capable of infecting others, and in some cases, causing paralysis. This is why 2 decades ago — with polio no longer an issue domestically — the U.S. switched to an all-Salk injectable polio vaccine regimen for children, away from a combination that used the Sabin oral polio vaccine (OPV) and the injectable polio vaccine. But many countries, especially those in which wild polio still circulates, still use OPV. * * *
In response, [U.S.] healthcare professionals and policymakers need to encourage widespread immunization in areas in which cVDPVs have been found. It is not surprising that this problem has been detected in the same locales in which a very large measles outbreak recently occurred. I suspect other areas likely have cVDPVs circulating as well, and broader wastewater surveillance coupled with targeted catch up immunization will be critical. I can’t stress this point enough: high vaccination rates are required for resiliency.
In related virus news, the Centers for Disease Control released a report titled “Hepatitis C Treatment Among Insured Adults — the United States, 2019–2020.”
What is already known about this topic?
Direct-acting antiviral (DAA) treatment is recommended for nearly all persons with hepatitis C and cures ≥95% of cases. Treatment saves lives, prevents transmission, and is cost saving.
What is added by this report?
Treatment rates are low overall and vary by age and insurance payor. DAA treatment is lowest among young adults aged 18–29 years and Medicaid recipients, and within Medicaid, among persons reporting Black or other race and persons in states with treatment restrictions.
What are the implications for public health practice?
Timely initiation of DAA treatment, regardless of insurance type, is critical to reducing viral hepatitis–related mortality, disparities, and transmission.
Also of note,
Across insurance types, ≥75% of persons treated initiated treatment within 180 days after diagnosis. The smaller percentage of persons treated within 180 days after diagnosis might indicate lack of access to a hepatitis C treatment provider, insurance denial, or loss to follow-up. Treatment coverage can be increased by providing integrated care, patient navigation, and care coordination (15). The introduction of simplified hepatitis C treatment algorithms reducing the number of laboratory tests and in-person visits can facilitate patient-centered treatment (20).
From the U.S. Healthcare business front
- The Food and Drug Administration issued “a final rule to improve access to hearing aids which may, in turn, lower costs for millions of Americans. This action establishes a new category of over-the-counter (OTC) hearing aids, enabling consumers with perceived mild to moderate hearing impairment to purchase hearing aids directly from stores or online retailers without needing a medical exam, prescription or a fitting adjustment by an audiologist.” The rule will take effect in 60 days / mid-October.
- STAT News reports Merck has cut a deal to enter the mRNA market.
On Tuesday, the company announced a deal to develop mRNA-based vaccines and therapies with Orna Therapeutics, a Cambridge-based startup that launchedduring the pandemic with a slightly different take on mRNA technology. Merck will pay Orna a $150 million upfront fee, invest $100 million in a new funding round for the company, and offer $3.5 billion in longer-term milestones.
- The American Hospital Association tells us.
Hospital patients are sicker and more medically complex than they were before the COVID-19 pandemic, driving up hospital costs for labor, drugs and supplies, according to a new AHA report.
“Combined with rapidly rising economy-wide inflation and reimbursement shortfalls, these mounting costs are threatening the financial stability of hospitals around the country,” the report notes.
Hospital patient acuity as measured by average length of stay (ALOS) rose almost 10% between 2019 and 2021, including a 6% increase for non-COVID-19 Medicare patients as the pandemic contributed to delayed and avoided care, the report notes. For example, ALOS rose 89% for patients with rheumatoid arthritis and 65% for patients with neuroblastoma and adrenal cancer. In 2022, patient acuity as reflected in the case mix index rose 11.1% for mastectomy patients, 15% for appendectomy patients and 7% for hysterectomy patients.
These increases have occurred at a time of significant financial challenges for hospitals and health systems, which have still not received support to address the delta and omicron surges that have comprised the majority of all COVID-19 admissions. As a result, AHA is asking Congress to halt its Medicare payment cuts to hospitals and other providers; extend or make permanent certain waivers that improve efficiency and access to care; extend expiring health insurance subsidies for millions of patients; and hold commercial insurers accountable for improper and burdensome business practices.