Weekend Update

Congress continues it floor and committee work this coming week. Committees are in engaged in organizational meetings on both sides of Congress. Senate Committees principally will be engaged in holding hearings on Presidential cabinet nomination.
The House Oversight and Reform Committee, whose jurisdiction includes the FEHBP, holds its organizational meeting tomorrow at 2 pm. The Chair will continue to be Rep. Carolyn Maloney (D NY) and the Ranking Member will be Rep. James Comer (R KY). The party ratio will be 25 Democrats and 20 Republicans.
Meanwhile, Bloomberg reports that
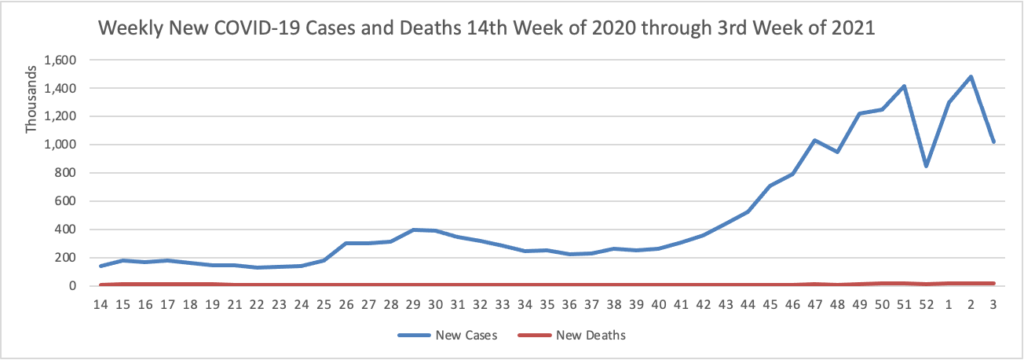
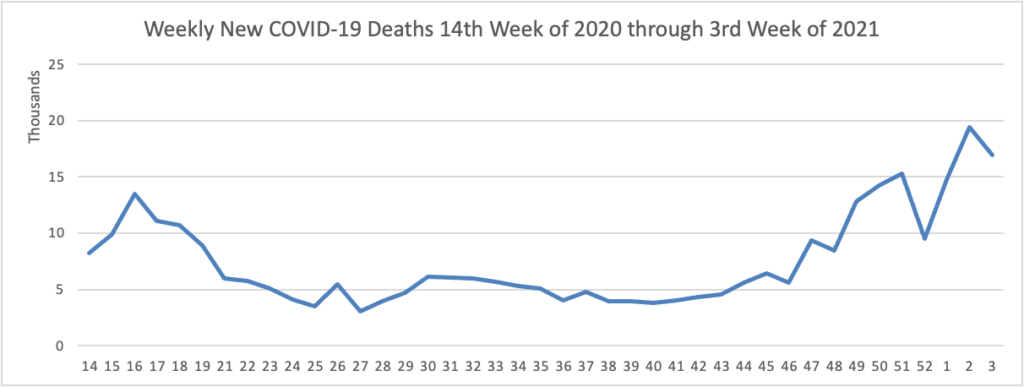
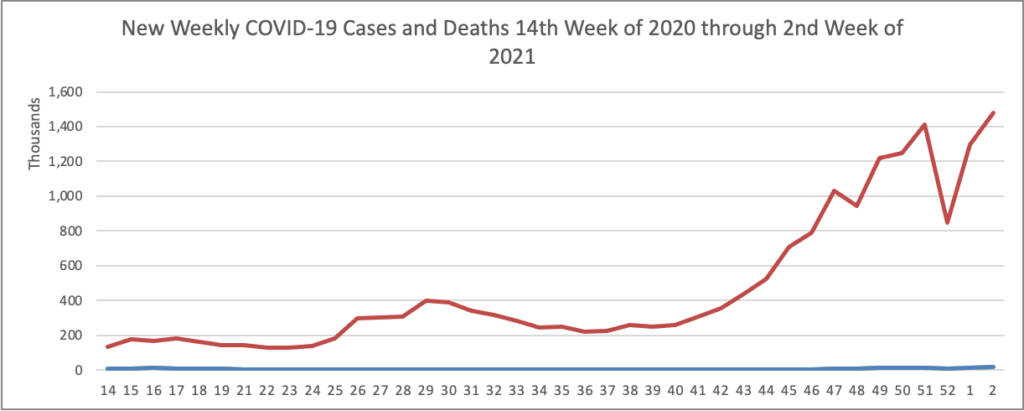
Joe Biden’s presidency began at a choreographed sprint, with a series of executive actions to erase Donald Trump’s legacy and reset the nation’s course. But as his first full week in office came to a close, the new president was discovering the limits of his power.
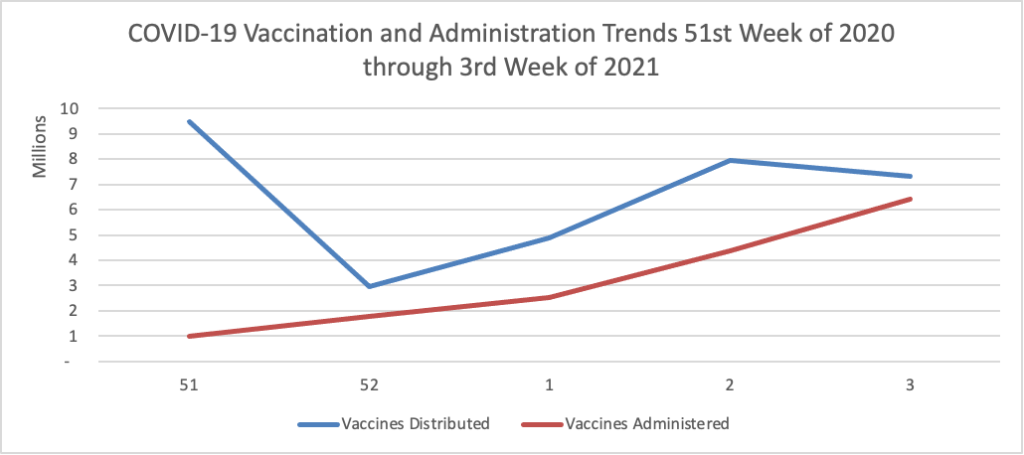
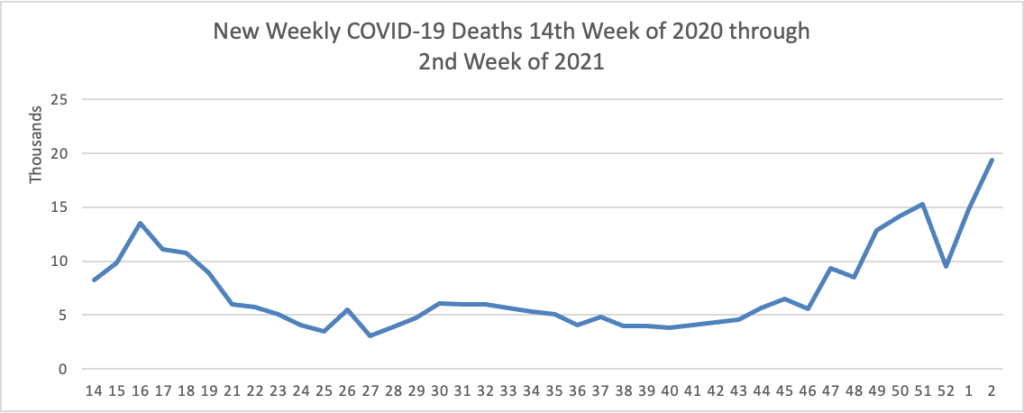
His administration’s efforts to bolster vaccine production ran into the same hurdles that plagued the Trump administration — bottlenecks both at factories and in clinics — and Biden’s advisers had to clean up after the president said any American would be able to get inoculated by the spring. * * *
[FEHBlog note — Per the CDC’s Vaccinations site, on average 1.3 million doses of COVID-19 vaccine were administered on Friday and Saturday.]
He’s meanwhile encountering familiar roadblocks in Congress, where just four of his cabinet nominees have been confirmed 12 days into his presidency, and he’s found no traction among congressional Republicans for another big stimulus bill. * * *
Ten GOP senators wrote to Biden on Sunday with an alternative proposal for a slimmed-down stimulus bill. The White House says it will review the offer. A smaller plan that passed with bipartisan support would leave Democrats free to pursue more contentious elements using a partisan budget tool.
The Wall Street Journal adds
The offer is the first Republicans have forwarded since Mr. Biden proposed the $1.9 trillion plan, which Republicans have said is too costly and includes unneeded initiatives, and tests whether the Biden administration and Democrats in Congress will seek compromise or try to pass the relief package themselves. Democratic leaders have said they plan to begin a legislative process that would bypass the need for Republican support this week, with the first step coming as soon as Monday.
FEHBlog Public Service Announcement: The Centers for Disease Control on Saturday implemented one of the President’s executive orders by requiring
the wearing of masks by all travelers into, within, or out of the United States, e.g., on airplanes, ships, ferries, trains, subways, buses, taxis, and ride-shares. The mask requirement also applies to travelers in U.S. transportation hubs such as airports and seaports; train, bus, and subway stations; and any other areas that provide transportation. Transportation operators must require all persons onboard to wear masks when boarding, disembarking, and for the duration of travel. Operators of transportation hubs must require all persons to wear a mask when entering or on the premises of a transportation hub.
This order will be effective on February 2, 2021. For more information on the Order or to view frequently asked questions, visit: https://www.cdc.gov/quarantine/masks/mask-travel-guidance.html