Weekend update

The House and Senate are on district/state work breaks until April 13.
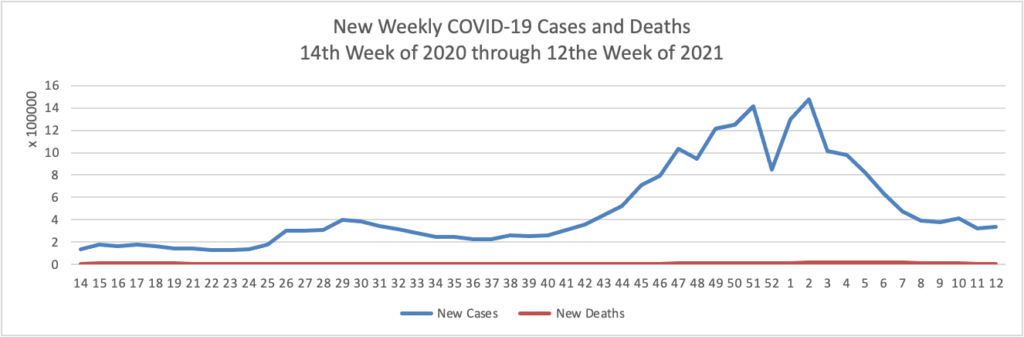
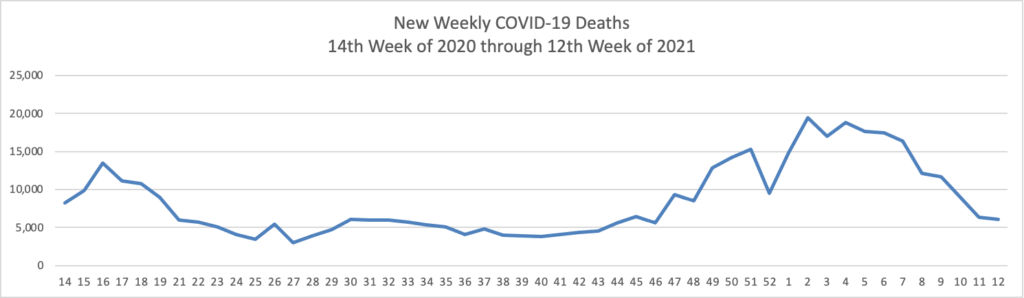
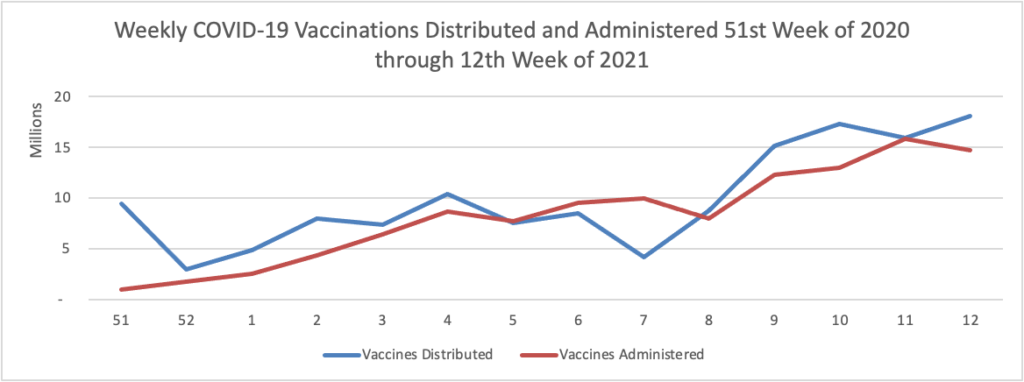
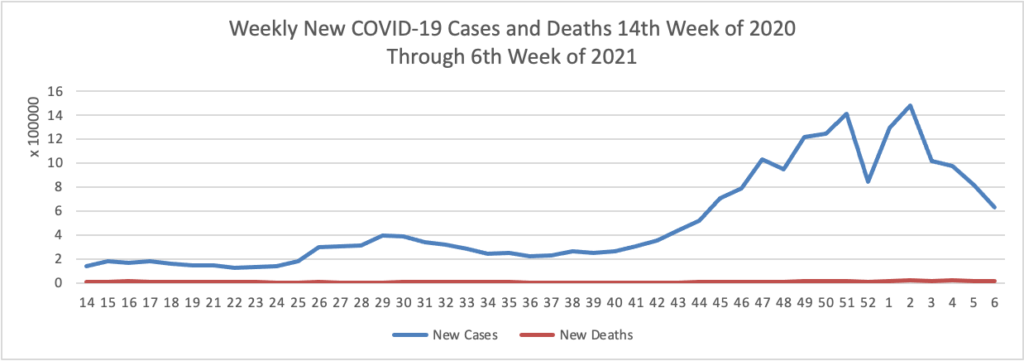
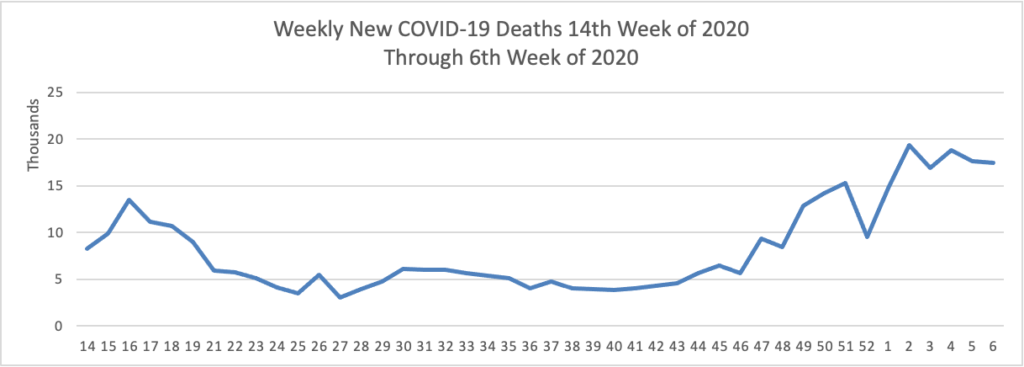
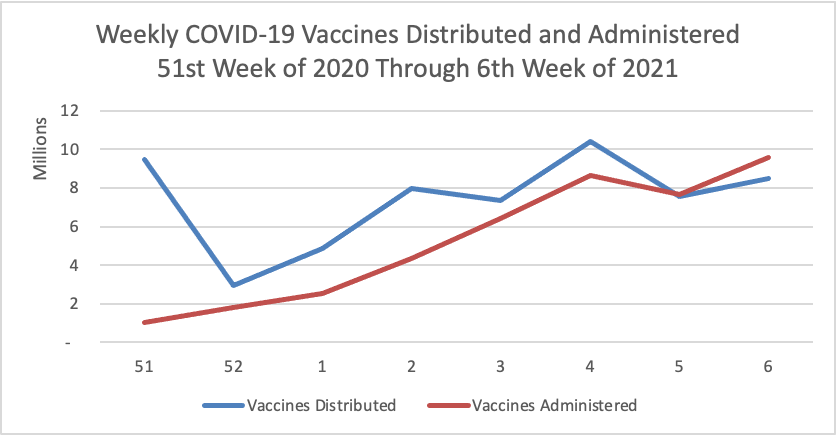
From the COVID-19 herd immunity front —
The FEHBlog always looks forward to the lead article in the Wall Street Journal’s weekend review section. Yesterday’s thought provoking article was headlined “Herd Immunity Won’t Save Us—but We Can Still Beat Covid-19 — Innovative contact tracing and just-in-time vaccination can get the pandemic under control—and prepare us for the next one.” The article, which was written by a group of epidemiologists, focuses on the worldwide eradication of smallpox which was quite an achievement in 1980 roughly 175 years after the smallpox vaccine was first administered. The article discusses the importance of the vaccine being able to prevent the disease in an infected, asymptomatic person which was the case with smallpox which had a 12 day incubation period.
Wikipedia notes
The 1947 New York City smallpox outbreak occurred in March 1947 and was declared ended on April 24, 1947. The outbreak marked two milestones for America. First, it was the largest mass vaccination effort ever conducted for smallpox in America, and second, it marked the last outbreak of smallpox in America. Within three weeks of the discovery of the outbreak, the U.S. Public Health Service, in conjunction with New York City health officials, had procured the smallpox vaccine and inoculated over 6,350,000 adults and children.[1]Of that number, 5,000,000 had been vaccinated within the first two weeks. The rapid response was credited with limiting the outbreak to 12 people, 10 of whom recovered, while 2 died.
The Wall Street Journal article explains that the COVID-19 vaccines also allow for just-in-time vaccinations:
Despite the rapid onset of viral infection (an average of 6 days after exposure, with a range of 2-14 days), Johnson & Johnson has reported success in preventing moderate to severe disease as early as 7 days after the administration of its single-dose vaccine. In a real-world study in Israel, the Pfizer vaccine also prevented severe disease soon after a single dose.
It is not too late to find, isolate and vaccinate those who do not yet have Covid-19 but are most likely to get it. It is not too late to use just-in-time vaccination to stop outbreaks in midcourse and prevent the spread of infection.
The Wall Street Journal in another herd immunity article reminds us that
“We definitely need to get kids vaccinated if we want to be as close to normal as we can,” said Octavio Ramilo, chief of infectious diseases at Nationwide Children’s Hospital, in Ohio. As governments push to move past the pandemic, vaccinating children is emerging as a key obstacle, along with initially limited supplies of vaccines.
Vaccines probably won’t be ready for use in younger children until early 2022, health experts said, in part because researchers need to test lower doses.
“The dose is not such a big leap to go from adults to teens,” said Katherine Luzuriaga, a pediatric infectious disease physician and the lead investigator of Moderna’s adolescent trial at the University of Massachusetts Medical School site. “Once we start going into the younger age groups, there’s a bit more work to determine the appropriate doses.”
As mentioned in Friday’s post, Pfizer and Moderna are testing on children. Vaccinating older high schoolers and college age kids which already is possible as the Pfizer vaccine is approved for persons over age 16 and the other two vaccines are available to persons over 18.
The FEHBlog believes that we should stay the current vaccination course until supply exceeds immediate demand likely in the next quarter of the year.
Vaccinating those at most risk of severe illness and death has been the first priority of vaccination drives. In most countries, that has meant giving priority to elderly citizens and those with conditions that heighten their risk of severe Covid-19.
The rate of hospitalization is 35 times as high, and the death rate is 1,100 times as high, among people 65 to 74 years old infected with Covid-19, compared with children ages 5 to 17, according to the U.S. Centers for Disease Control and Prevention.
Health authorities say children don’t need to be vaccinated to start resuming certain activities like in-person learning at schools that are taking precautionary measures. Some experts caution against focusing too heavily on a specific herd immunity target, as building up population-level protection is an incremental process.
From the medical innovation front (non-COVID) —
- Yesterday the Food and Drug Administration announced that marketing approval for
Abecma (idecabtagene vicleucel), a cell-based gene therapy to treat adult patients with multiple myeloma who have not responded to, or whose disease has returned after, at least four prior lines (different types) of therapy. Abecma is the first cell-based gene therapy approved by the FDA for the treatment of multiple myeloma.
“The FDA remains committed to advancing novel treatment options for areas of unmet patient need,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “While there is no cure for multiple myeloma, the long-term outlook can vary based on the individual’s age and the stage of the condition at the time of diagnosis. Today’s approval provides a new treatment option for patients who have this uncommon type of cancer.”
- On Friday the FDA announced other hopeful news
The U.S. Food and Drug Administration approved the first in the world non-surgical heart valve to treat pediatric and adult patients with a native or surgically-repaired right ventricular outflow tract (RVOT), the part of the heart that carries blood out of the right ventricle to the lungs. The device is designed for patients who have severe pulmonary valve regurgitation (blood leaking backward into the right lower chamber of the heart), a condition that often results from congenital heart disease (CHD). The device, called the Harmony Transcatheter Pulmonary Valve (TPV) System, is intended to improve blood flow to the lungs in patients with severe pulmonary valve regurgitation without open-heart surgery, which is the current standard of care. The use of the Harmony valve may delay the time before a patient needs additional open-heart surgery. It can also potentially reduce the total number of open-heart surgeries required over an individual’s lifetime.
CHDs affect nearly 1% of―or about 40,000―births per year in the United States.