Friday Stats and More
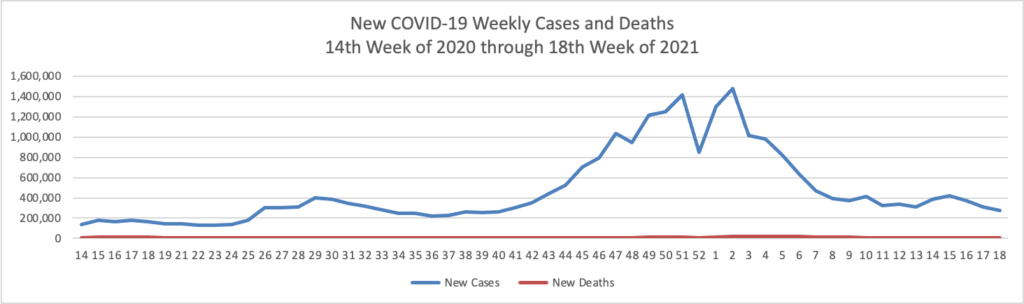
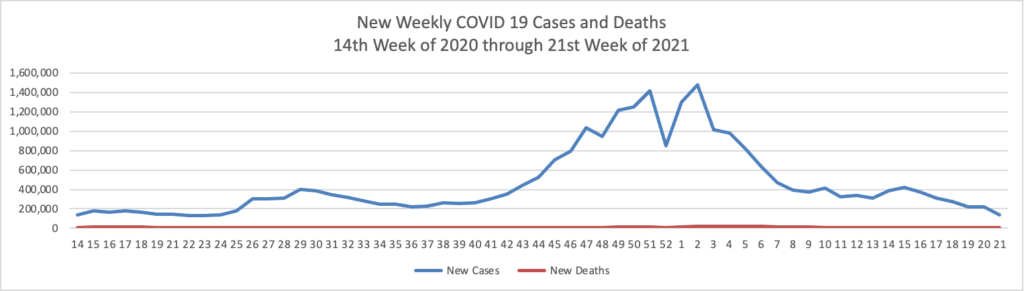
Based on the Centers for Disease Control’s COVID-19 Data Tracker website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through 21st week of this year (beginning April 2, 2020, and ending May 26, 2021; using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

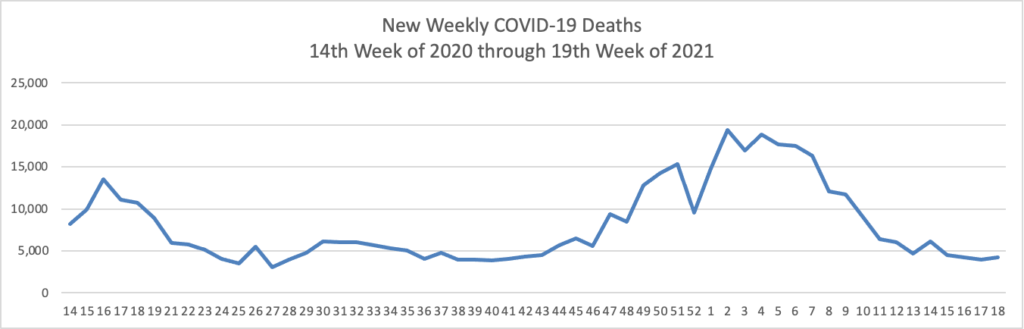
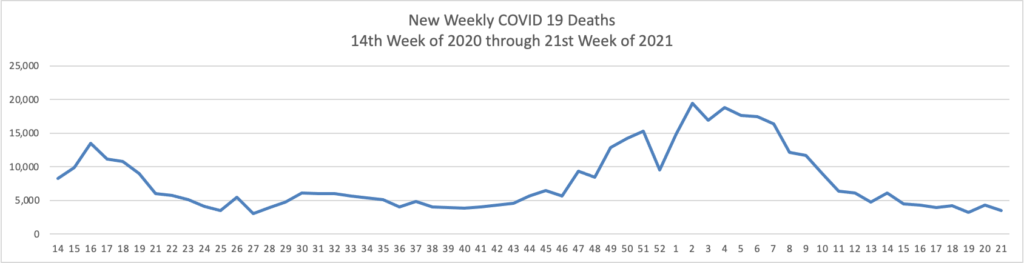
The FEHBlog has noticed that the new cases and deaths chart shows a flat line for new weekly deaths because new cases significantly exceeds new deaths. Accordingly here is a chart of new COVID-19 deaths over the period (April 2, 2020, through May 26, 2021):
Finally here is a COVID-19 vaccinations chart over the period December 17, 2020, through May 26, 2021 (five months) which also uses Thursday as the first day of the week:
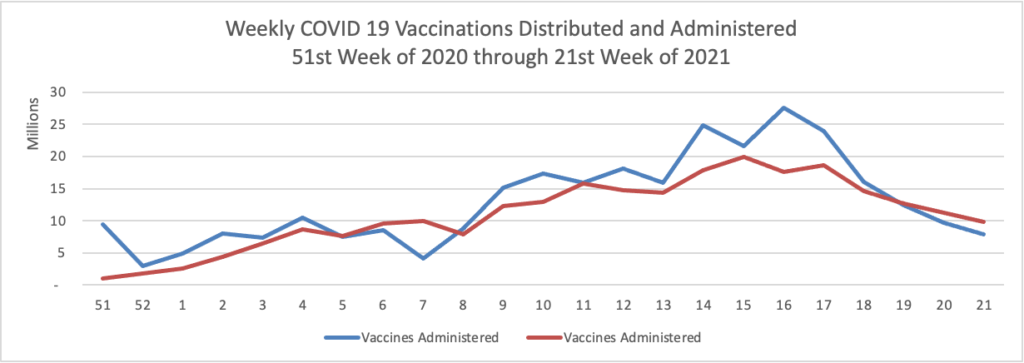
The Centers for Disease Control observes that “COVID-19 cases and deaths in the United States have dropped to their lowest levels in nearly a year, and the number of people vaccinated continues to grow. As of May 27, 2021, nearly 133 million people in the U.S. are fully vaccinated, and the national percentage of COVID-19 tests that came back positive over the last 7 days was less than 3%”
Also on the COVID-19 vaccine front, Healthcare Dive reports that
Federal equal employment opportunity laws do not prohibit policies requiring that all employees who physically enter a workplace receive a COVID-19 vaccination, so long as such policies comply with the reasonable accommodation provisions of the Americans with Disabilities Act and Title VII of the Civil Rights Act as well as other applicable laws, according to technical assistance from the U.S. Equal Employment Opportunity Commission updated May 28.
Title VII and the ADA require employers to provide reasonable accommodations for employees who, because of a disability or a sincerely held religious belief, practice, or observance, do not get vaccinated for COVID-19, unless providing an accommodation would pose an undue hardship on the operation of the employer’s business, EEOC said. Employers with such a requirement also may need to respond to allegations that the requirement has a disparate impact on, or disproportionately excludes, an employee based on protected characteristics including age, race, color, religion, sex and national origin.
Employers also may offer incentives to employees to voluntarily show documentation or confirmation that they have received a COVID-19 vaccine, but the agency outlined some limits in the event that employers are incentivizing employees to voluntarily receive a vaccine administered by an employer or its agent. An employer may offer an incentive to employees to provide documentation or other confirmation from a third party not acting on the employer’s behalf, such as a pharmacy or health department, that employees or their family members have been vaccinated.
In other news —
- Today, the President released his Fiscal Year 2022 U.S. budget. Here is a link to the Office and Management and Budget’s fact sheet. OPM’s budget information may be found beginning on page 1211 of the Appendix.
- Yesterday, the Milliman consulting firm released its 2021 Medical Index. Peering into its crystal ball, Milliman states that “We project healthcare costs will grow by approximately 8.4% for the MMI family from 2020 to 2021. This rate, driven by a forecasted rebound in healthcare utilization, is higher than historical healthcare cost increases and gross domestic product (GDP) growth over the past five years.”
- Healthcare Dive informs us that “Despite the financial turmoil COVID-19 wrought on U.S. medical practices over the past year, physician income has remained relatively steady, according to a new survey by the Medical Group Management Association. The survey — which included 185,000 providers among more than 6,700 physician-owned and hospital-owned practices — concluded they experienced flat or modest income growth in 2020. While compensation for primary care physicians — traditionally one of the lowest-paid specialties — grew, most specialist physicians either experienced small bumps or decreases in their income. Many medical specialties also experienced a decrease in patient encounters last year — a nearly inevitable outcome after many elective procedures were postponed in order to keep hospital capacity low enough to treat COVID-19 patients. However, the MGMA survey concluded that most practices saw patient volumes return to normal by mid-summer of 2020. Analysts predict the trend will continue to grow in 2021.” Agreed.
- Recycle Intelligence informs us that “Hospital revenue, volumes, and margins increased in April 2021 both year-to-date and year-over-year, but have a long way to go in terms of COVID-19 recovery, according to a report by health care consulting firm Kaufman Hall. Despite the increases, hospital financial performance is down compared to last month.”
- Last but not least, the Wall Street Journal reports that “A pathbreaking pill for lung cancer from Amgen Inc. was approved by the U.S. Food and Drug Administration, adding a new potential blockbuster to the biotech giant’s aging stable of drugs. The drug, called Lumakras, was approved Friday to treat a portion of lung cancer patients with a particular genetic mutation who have already tried other therapies. The mutation, known as KRAS, is among the most common found in cancers, but researchers struggled for so many years to find a medicine that can treat it that the mutation came to be considered ‘undruggable.’” Bravo. The Journal adds that “The company will charge $17,900 a month for the drug in the U.S., an Amgen spokeswoman said. Analysts project the drug could eventually ring up more than $1 billion in annual sales.”