Monday Roundup

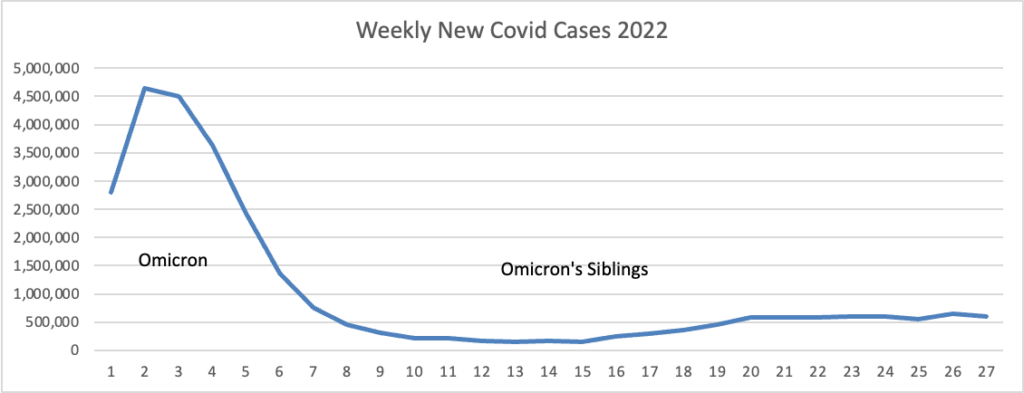
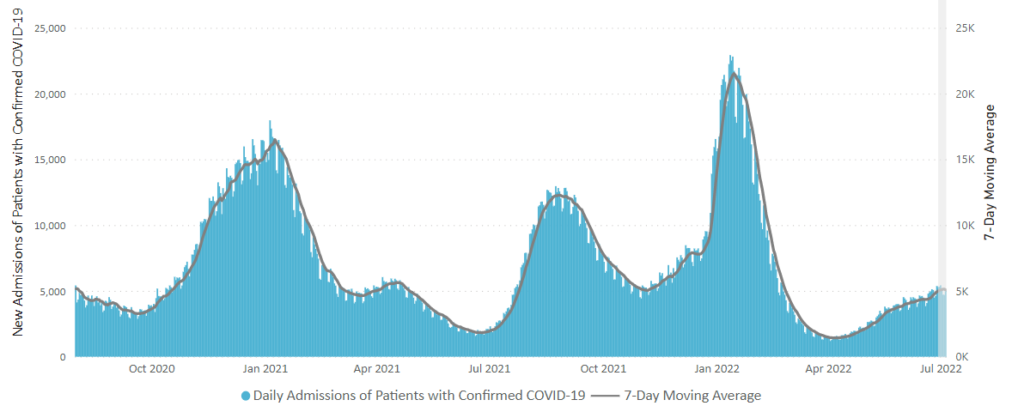
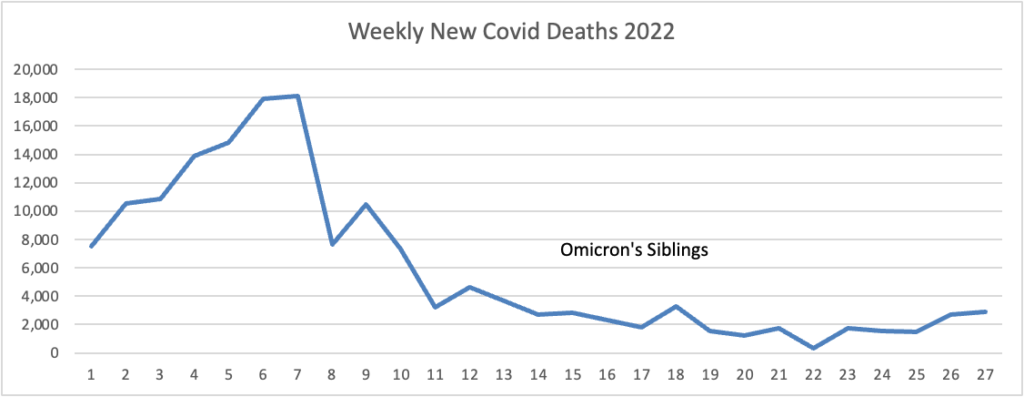
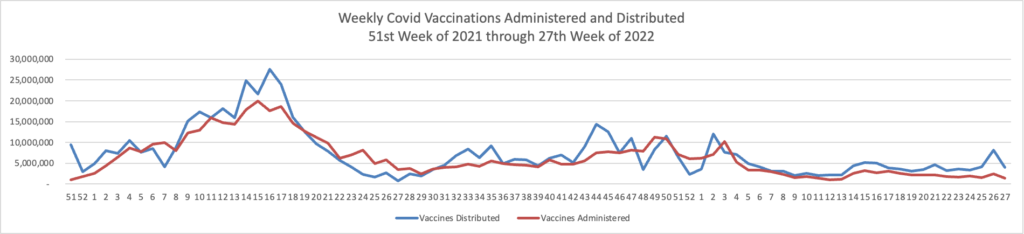
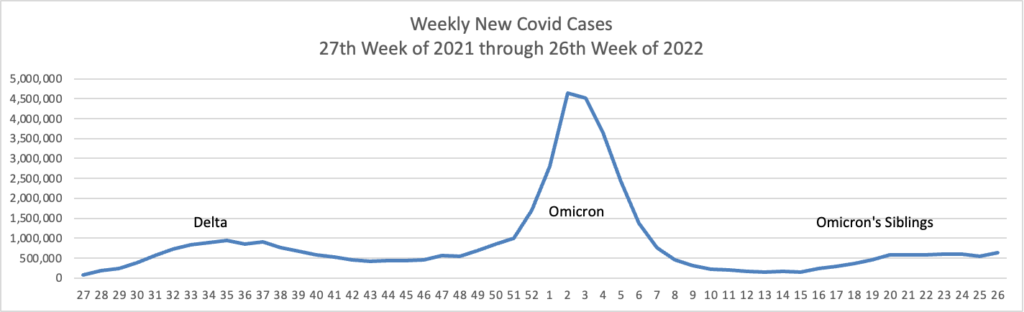
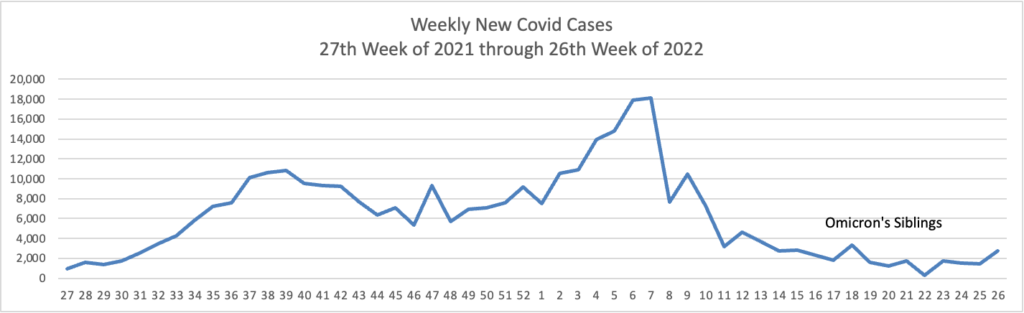
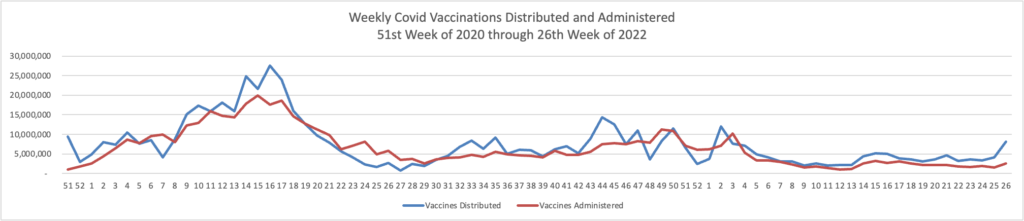
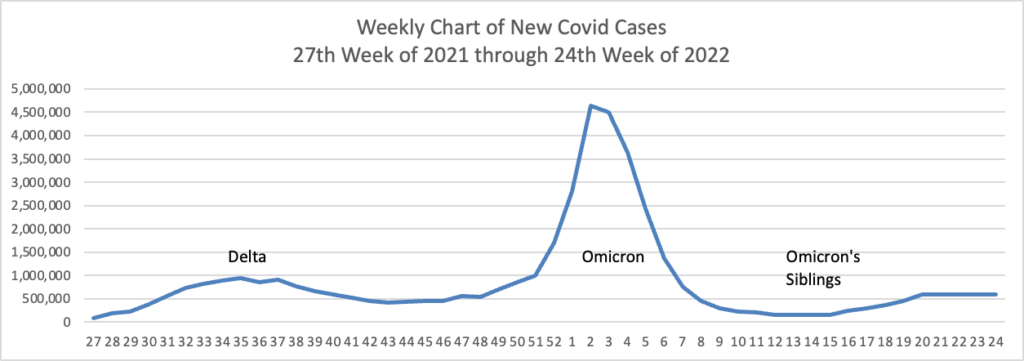
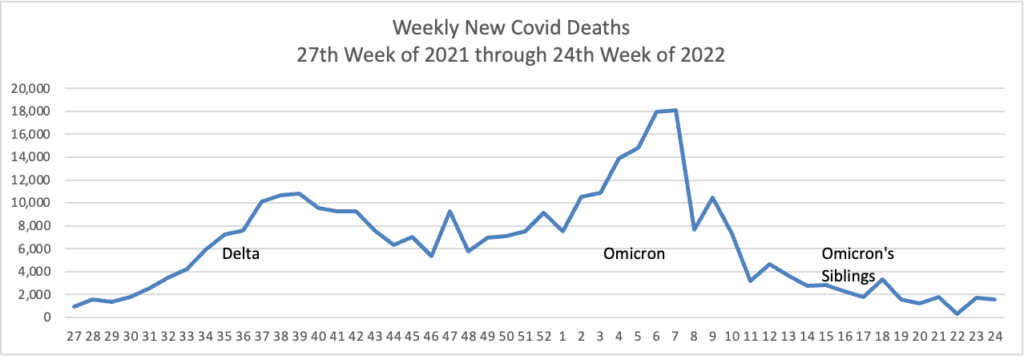
From the Omicron and siblings front, STAT News reports
The Biden administration is preparing a sweeping initiative to develop a next generation of Covid-19 immunizations that would thwart future coronavirus variants and dramatically reduce rates of coronavirus infection or transmission, building on current shots whose impact has been mainly to prevent serious illness and death, the White House told STAT.
To kick off the effort, the White House is gathering key federal officials, top scientists, and pharmaceutical executives including representatives of Pfizer and Moderna for a Tuesday “summit” to discuss the new technologies and lay out a road map for developing them.
“These are vaccines that are going to be far more durable, that are going to provide far longer-lasting protection, no matter what the virus does or how it evolves,” Ashish Jha, the White House Covid-19 response coordinator, said in an interview. “If we can drive down infections by 90% … Covid really begins to fade into the background, and becomes just one more respiratory illness that we have to deal with.”
Here’s hoping. Curiously, the federal government did not start this effort last year when Delta was raging.
The Centers for Disease Control posted an information sheet on the Novovax vaccine. The FEHBlog was surprised to read “Novavax COVID-19 vaccine is not authorized for use as a booster dose.” The FEHBlog had read months ago that the Novovax vaccine would make a dandy booster to a series of mRNA shots.
Medscape discusses long Covid symptoms.
People who reported sore throats, headaches, and hair loss soon after testing positive for COVID-19 may be more likely to have lingering symptoms months later, according to a recent study published in Scientific Reports.
Researchers have been trying to determine who faces a higher risk for developing long COVID, with symptoms that can last for weeks, months, or years after the initial infection. So far, the condition has been reported in both children and adults, healthy people, those with preexisting conditions, and a range of patients with mild to severe COVID-19.
“These people are not able to do necessarily all the activities they would want to do, not able to fully work and take care of their families,” Eileen Crimmins, PhD, the senior study author and a demographer at the University of Southern California’s Leonard Davis School of Gerontology, told the Los Angeles Times.
“That’s an aspect of this disease that needs to be recognized because it’s not really as benign as some people think,” she said. “Even people who have relatively few symptoms to start with can end up with long COVID.”
From the Affordable Care Act front, the Department of Health and Human Services released today a proposed third version (Obama, Trump, Biden) version of a rule implementing Section 1557, the individual non-discrimination provision of the Affordable Care Act. Here are links to the proposed rule and the Department’s fact sheet. The law needs an implementing rule because its wording is garbled. The FEHBlog didn’t think he would ever see a more complicated rule than the Obama Administration’s 2016 rule, but at least at first glance, it appears that the Biden Administration has cleared that high bar. Later this week, the FEHBlog will offer his take on the extent of the rule’s application to the FEHBP. The public comment period will be 60 days long once the proposed rule is published in the Federal Register.
The Wall Street Journal has launched an investigative journalism campaign against certain large charitable hospitals.
Nonprofit hospitals get billions of dollars in tax breaks in exchange for providing support to their communities. A Wall Street Journal analysis shows they are often not particularly generous.
These charitable organizations, which comprise the majority of hospitals in the U.S., wrote off in aggregate 2.3% of their patient revenue on financial aid for patients’ medical bills. Their for-profit competitors, a category including publicly traded giants such as HCA Healthcare Inc., wrote off 3.4%, the Journal found in an analysis of the most-recent annual reports hospitals file with the federal government.
Among nonprofits with the smallest shares of patient revenue going toward charity care—well under 1%—were high-profile institutions including the biggest hospitals of California’s Stanford Medicine and Louisiana’s Ochsner Health systems. At Avera Health, a major hospital system in South Dakota, charity care was roughly half of 1% of patient revenue across all its 18 hospitals.
You get the gist. These Journal investigations usually attract attention on Capitol Hill.
From the public health front, the Washington Post discusses the meaning of a pre-diabetes diagnosis to the over 65 crowd.
More than 26 million people 65 and older have prediabetes, according to the Centers for Disease Control and Prevention. How concerned should they be about progressing to diabetes?
Not very, some experts say. Prediabetes — a term that refers to above-normal but not extremely high blood sugar levels — isn’t a disease, and it doesn’t imply that older adults who have it will inevitably develop Type 2 diabetes, they say.
“For most older patients, the chance of progressing from prediabetes to diabetes is not that high,” said Robert Lash, the chief medical officer of the Endocrine Society. “Yet labeling people with prediabetes may make them worried and anxious.”
Other experts believe it is important to identify prediabetes, especially if doing so inspires older adults to add more physical activity, lose weight and eat healthier diets to help bring their blood sugar under control.
Based on personal experience, the FEHBlog finds himself supporting “the other experts.”
From the OPM / FEHB front —
- OPM today issues a fact sheet on the steps being taken to implement the President’s June 2021 Executive Order on increasing diversity, equity, and inclusion in the federal workforce.
- FedSmith has an article encouraging federal annuitants to take look this Open Season at FEHB plans which offer an integrated Medicare Advantage. The number of those plans has been growing over the past two Open Seasons and the FEHBlog anticipates the number will continue to grow this coming Open Season.
From the telehealth front, mHealth Intelligence points out
The benefits of breastfeeding are well-documented. Breast milk is a comprehensive source of infant nutrition, can help stave off some short-and long-term illnesses, and enables babies to gain valuable antibodies from their mothers, according to the Centers for Disease Control and Prevention. Further, breastfeeding can reduce the mother’s risk of developing several conditions, including breast and ovarian cancer and type 2 diabetes.
Though a majority of babies are breastfed initially, there appears to be a drop-off at the six-month mark, and rates continue to decline from there. In total, about 84 percent of babies were breastfed in 2018, but only 57 percent were breastfed at six months and 35 percent at 12 months, according to CDC data.
To support breastfeeding, the five-hospital Trinity Health of New England system joined forces with Nest Collaborative last month to launch a telehealth program.
The telehealth-enabled breastfeeding support program, launched at the end of June, connects pregnant women and new mothers to a nationwide network of lactation consultants.
“Nest Collaborative’s [lactation consultants] help families reach their breastfeeding goals and assist them in making informed decisions about infant feeding options,” said Judith Nowlin, CEO of Nest Collaborative, in an email.