Tuesday’s Tidbits

From Washington DC, the Wall Street Journal reports
- “President Biden and House Speaker Kevin McCarthy remained at loggerheads after a meeting Tuesday at the White House, appearing to make little progress in averting the first-ever default by the federal government as soon as next month.
- “House Republicans have demanded deep spending cuts in exchange for raising the debt ceiling and criticized Mr. Biden for not starting talks earlier. But Mr. Biden and Democrats in Congress maintain that the federal borrowing limit should be raised without preconditions and have called the GOP stance irresponsible. Neither side has presented a path forward that could win enough support to pass both chambers of Congress.
- ”I didn’t see any new movement,” Mr. McCarthy said after leaving the meeting. He said he thought negotiators only had about two weeks to reach an agreement. He said there were staff-level meetings planned and the key leaders would meet again on Friday.”
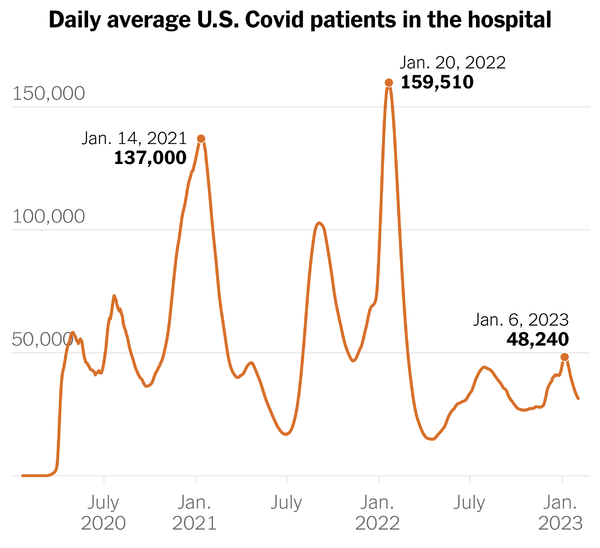
From the end of the public health emergency front —
- The Department of Health and Human Services released a fact sheet on the end of the Covid public health emergency, which ends on Thursday, May 11.
- The Washington Post tells us,
- “The federal government will allow doctors to keep using telemedicine to prescribe certain medications for anxiety, pain and opioid addiction, extending for six months emergency flexibilities established during the coronavirus pandemic.
- “The Drug Enforcement Administration and Substance Abuse and Mental Health Services Administration made the announcement Tuesday, two days before the telemedicine flexibilities were set to expire along with the coronavirus public health emergency.
- “The ability to prescribe controlled medications remotely will run through Nov. 11, 2023. And that deadline will be longer still if doctors have already established a telemedicine relationship with patients. In that circumstance, physicians can keep prescribing the medications virtually through Nov. 11, 2024.”
- Govexec informs us
- “President Biden on Tuesday officially revoked the COVID-19 vaccine mandates for federal employees and contractors that had already been mired in lawsuits that prevented them from being enforced.
- “The mandates–issued in September 2021–will end on May 12, Biden said in an executive order. The move had been expected following an announcement from the White House earlier this month, and will coincide with the end of the COVID public health emergency on May 11.”
- STAT News adds
- “The White House isn’t quite ready to launch its new pandemic response office for a neat handoff at the end of the Covid-19 public health emergency, White House Covid-19 Response Coordinator Ashish Jha told reporters Tuesday.
- “Jha said White House officials are in the middle of setting up an Office of Pandemic Preparedness and Response Policy that Congress mandated them to create in December, but it won’t be ready in time for a clean transfer at the end of the public health emergency on May 11.
- “He deflected questions about whether he will stay on after the transition.
From the substance abuse disorder front, Google tells us that this is National Fentanyl Awareness Day, and Shatterproof addresses four myths about fentanyl.
From the preventive services front, the U.S. Preventive Services Task Force posted
- “a draft recommendation statement on screening for breast cancer. The Task Force now recommends that all women get screened for breast cancer every other year starting at age 40. This is a B grade. More research is needed on whether or not women with dense breasts should have additional screening with breast ultrasound or MRI, and on the benefits and harms of screening in women older than 75. These are I statements.”
- The public comment period ends on June 5, 2023.
From the litigation front, STAT News reports
- “A federal jury handed a major win to Gilead Sciences on Tuesday in a closely watched battle with the U.S. government over the rights to groundbreaking HIV prevention pills.
- “The jury decided Gilead did not infringe on patents held by the Centers for Disease Control and Prevention and, in fact, that the agency’s patents were invalid. The CDC helped fund academic research into HIV prevention that later formed the basis for the pills. The Department of Health and Human Services contended that Gilead refused to reach a licensing agreement despite several attempts to reach a deal.
- “For its part, the company argued that it invented the pills — an older one called Truvada and a newer, upgraded version called Descovy — and that the concept of using Truvada to prevent HIV was well-known by the time the government tried to obtain its patents. Moreover, Gilead maintained that it acted in good faith during its negotiations with the government.”
From the tidbits front —
- Federal News Network relates
- “The Postal Service is falling short of its goal to start turning around its financial losses this year, but Postmaster General Louis DeJoy says the agency is taking “aggressive actions” to get the agency back on track to break even by the end of the decade.
- “USPS reported a $2.5 billion net loss for the second quarter of fiscal 2023, and is expected to see a net loss for the entire fiscal year.
- The agency saw more than an 8% decline in first-class mail volume and a 5% decline in package volume, compared to the same period last year.”
- OPM announced
- “U.S. Office of Personnel Management (OPM) Director Kiran Ahuja will deliver the commencement address to the 2023 graduating class of the University of Georgia’s (UGA) School of Public and International Affairs at the Ramsey Auditorium on the UGA campus.
- “Director Ahuja, an alumna of the University of Georgia School of Law, will speak to the Class of 2023 on the opportunities that a career in federal service offers. As federal agencies seek to fill the positions necessary to implement legislation such as the 2021 Bipartisan Infrastructure Law, OPM is leading the federal government’s recruitment efforts. Director Ahuja’s message to graduates will be simple: if you want a career with impact, the federal government is hiring.”
- HUB International points out that
- “The IRS recently released a Chief Counsel Memo confirming its long-standing position that all flexible spending account (“FSA”) expenses must be substantiated. This means that, no matter how small, each expense must have some kind of third-party verification. While Chief Counsel Memos are not official, binding IRS guidance, they are informative of the IRS’s views in a particular area.”
- Last Wednesday, “the FDA published a new web page with details about over-the-counter (OTC) Hearing Aids: What You Should Know before and after buying an OTC hearing aid.”