Thursday Miscellany

From the COVID-19 front
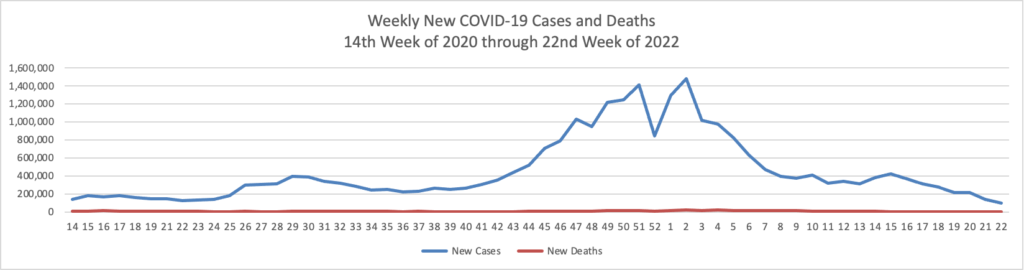
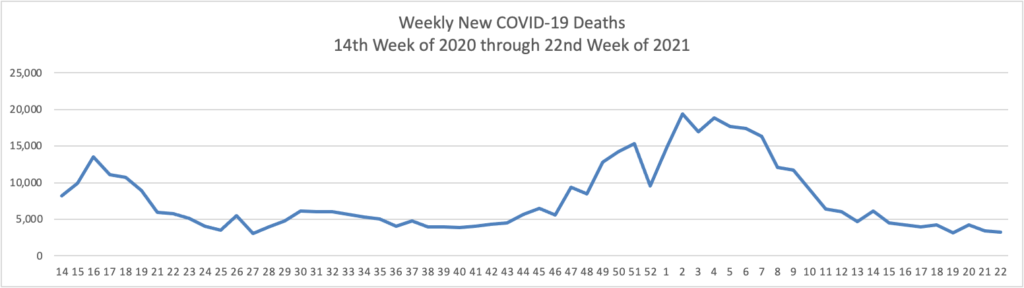
- Fierce Healthcare reports that “The quick rollout of the COVID-19 vaccine in the U.S. saved an estimated 279,000 lives and prevented 1.25 million hospitalizations, a new study finds. The study, released Wednesday, warns, however, that surges of new cases due to the highly transmissible delta variant could reverse these gains. “Until a greater majority of Americans are vaccinated, many more people could still die from this virus,” said Alison Galvani, Ph.D., director of the Yale Center for Infectious Disease Modeling and Analysis, which conducted the study alongside the Commonwealth Fund.”
- The Wall Street Journal reports that “Children are at extremely slim risk of dying from Covid-19, according to some of the most comprehensive studies to date, which indicate the threat might be even lower than previously thought. Some 99.995% of the 469,982 children in England who were infected during the year examined by researchers survived, one study found. In fact, there were fewer deaths among children due to the virus than initially suspected. Among the 61 child deaths linked to a positive Covid-19 test in England, 25 were actually caused by the illness, the study found.”
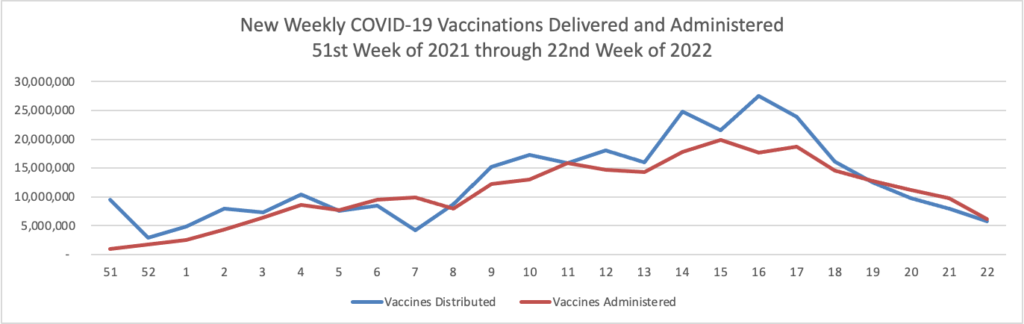
- The Journal also informs us that “Pfizer Inc. will seek clearance from U.S. regulators in coming weeks to distribute a booster shot of its Covid-19 vaccine to heighten protection against infections as new virus strains rise. The company said also it plans to start clinical trials in August of an updated version of its vaccine that would better protect against the Delta variant.” While the FEHBlog looks forward to lining up for the booster, Axios reports that “People who are fully vaccinated against the coronavirus do not need a booster shot at this time, the Food and Drug Administration and the Centers for Disease Control and Prevention said in a joint statement released Thursday evening.” Axios adds
One dose of the Pfizer-BioNTech or AstraZeneca coronavirus vaccine “barely” protects against the Delta variant of the virus, because of mutations the variant has developed, a new study published in the journal Nature Thursday found.
But two doses of those vaccines generated a neutralizing response to the variant in 95% of people, highlighting the importance of full vaccination against COVID-19, Axios’ Jacob Knutson writes.
- Bloomberg discusses the idea of offering COVID-19 vaccines at Dollar General stores. “The researchers found that in the most vulnerable decile, the number of retail pharmacies that are eligible to provide vaccines through the Federal Retail Pharmacy Program is the lowest. But these vulnerable regions are also where Dollar General and other discount stores like it tend to cluster.” It’s worth a shot?
The National Institutes of Health released its annual joint report on cancer mortality. “The report shows a decrease in death rates for 11 of the 19 most common cancers among men, and for 14 of the 20 most common cancers among women, over the most recent period (2014-2018). Although declining trends in death rates accelerated for lung cancer and melanoma over this period, previous declining trends for colorectal and female breast cancer death rates slowed and those for prostate cancer leveled off. Death rates increased for a few cancers like brain and other nervous system and pancreas in both sexes, oral cavity and pharynx in males, and liver and uterus in females.” STAT News points out
Accelerating declines in lung cancer deaths may account for much of the overall progress seen in recent years, the authors of the report said. Over the past two decades, the death rate for lung cancer has declined even faster than the rate at which patients are diagnosed with the disease. And while part of the early success in preventing lung cancer can be attributed to the massive drop in smoking rates, the authors note the most recent downward trends seem to correspond with the approval of new treatments for non-small cell lung cancer that improved the likelihood of survival.
Death rates from melanoma also saw an accelerated decline in the past decade, despite a growing number of diagnoses. Like in lung cancer, authors point to the introduction of novel treatments around the same time as the turnaround on the death rate. New targeted and immune checkpoint inhibitors were approved by the Food and Drug Administration in 2011, one year before major declines in death rates were seen in women and two years before they were seen in men.
On the prescription drug front
- The New York Times reports that “Under fire for approving a questionable drug for all Alzheimer’s patients, the Food and Drug Administration on Thursday greatly narrowed its previous recommendation and is now suggesting that only those with mild memory or thinking problems should receive it. The reversal, highly unusual for a drug that has been available for only a few weeks, is likely to reduce the approximate number of Americans who are eligible for the treatment to 1.5 million from six million.”
- GoodRX points out and discusses the fifteen most addictive prescription drugs and resource available to help the addicted. The National Institute on Drug Abuse has an outreach website for teenagers, for example.
In other healthcare news
Health Affairs blog bangs the drum for Congress to fund a universal patient identifier. For the reasons explained in the article, this step called for in the HIPAA statute of 1996 is long overdue.
Healthcare Dive reports that “Telehealth claim lines as a percentage of all medical claims dropped 13% in April, marking the third straight month of declines, according to new data from nonprofit Fair Health. The dip was greater than the drop of 5.1% in March, but not as large as the decrease of almost 16% in February. However, overall utilization remains significantly higher than pre-COVID-19 levels. The decline appears to be driven by a rebound in in-person services, researchers said. Mental health conditions bucked the trend, however, as the percentage of telehealth claim lines associated with mental conditions — the No. 1 telehealth diagnosis — continued to rise nationally and in every U.S. region.” The FEHBlog considers that to be good news because telehealth at least currently is best suited for mental health care and out of schedule healthcare situations.
In closing, the FEHBlog wants to emphasize an important aspect of last Thursday’s No Surprise Billing rule. As explained in the government’s model consumer notice for use by health plans,
When you get services from an in-network hospital or ambulatory surgical center, certain providers there may be out-of-network. In these cases, the most those providers may bill you is your plan’s in-network cost-sharing amount. This applies to emergency medicine, anesthesia, pathology, radiology, laboratory, neonatology, assistant surgeon, hospitalist, or intensivist services. These providers can’t balance bill you and may not ask you to give up your protections not to be balance billed.
If you get other services at these in-network facilities, out-of-network providers can’t balance bill you, unless you give written consent and give up your protections.”
The vast majority of surprise bills stem from out-of-network service provided by emergency rooms, air ambulance, and the types of providers listed above, all of whom are locked into using negotiation and baseball arbitration with the health plan. The only doctors who can approach the patient for a balancing billing waiver are the surgeon or oncologist in a non-emergency setting who meets with the patient well before the surgery. That makes sense.
This approach, however, will promote use of the independent dispute resolution system which the tri-agencies will unveil October 1. Three months is more than ample time for the FEHBlog’s fellow lawyer to prepare for this new business opportunity. Health plans should make sure that their out of network pricing negotiators are adequately staffed.