Monday Roundup

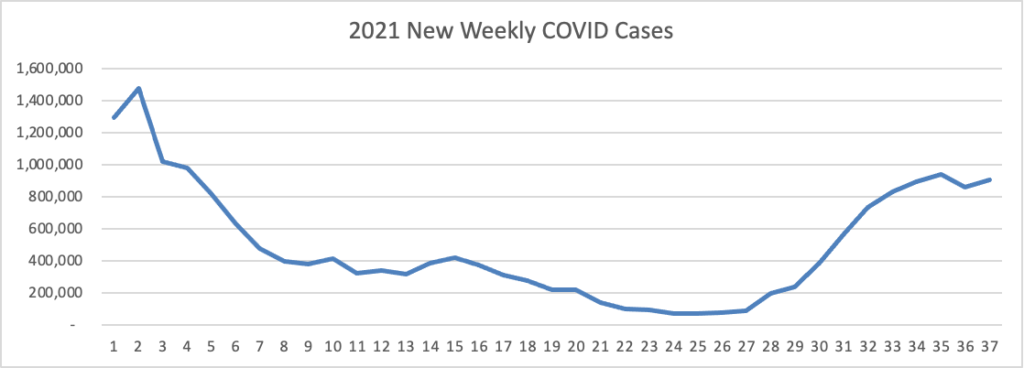
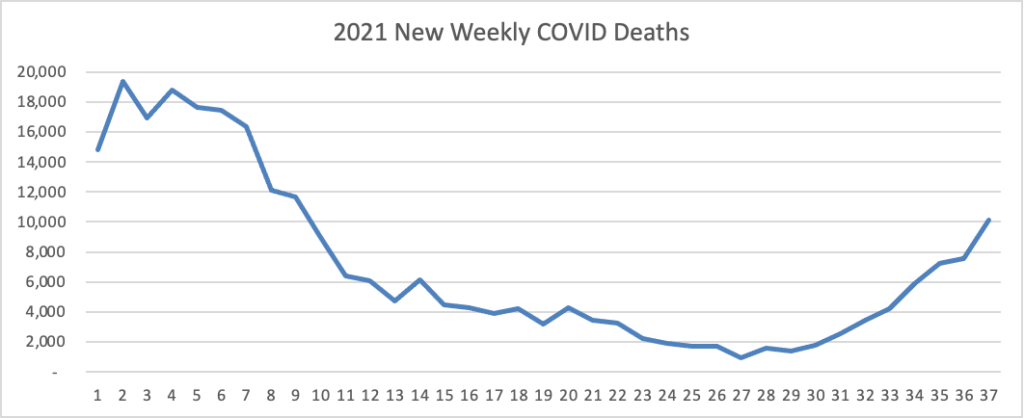
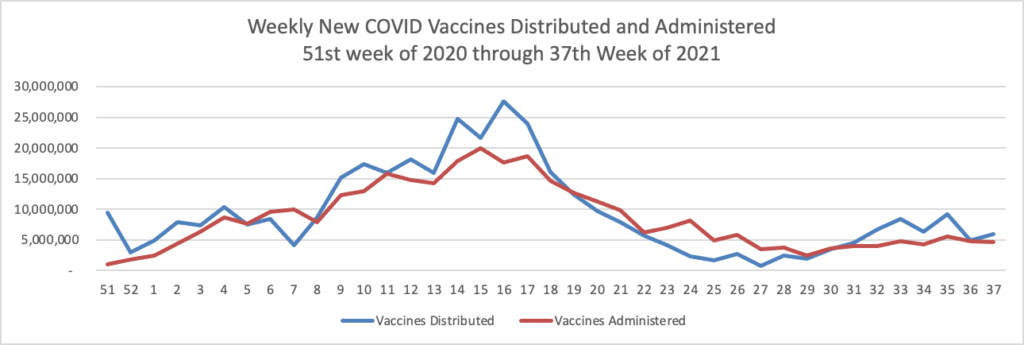
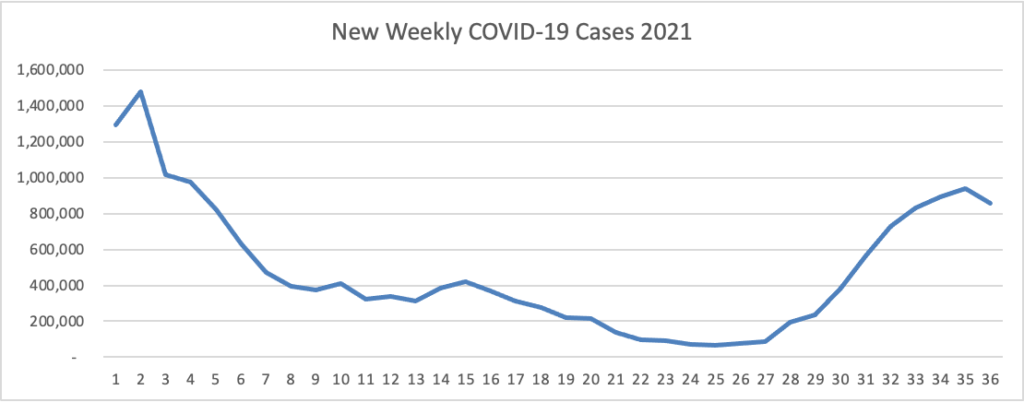
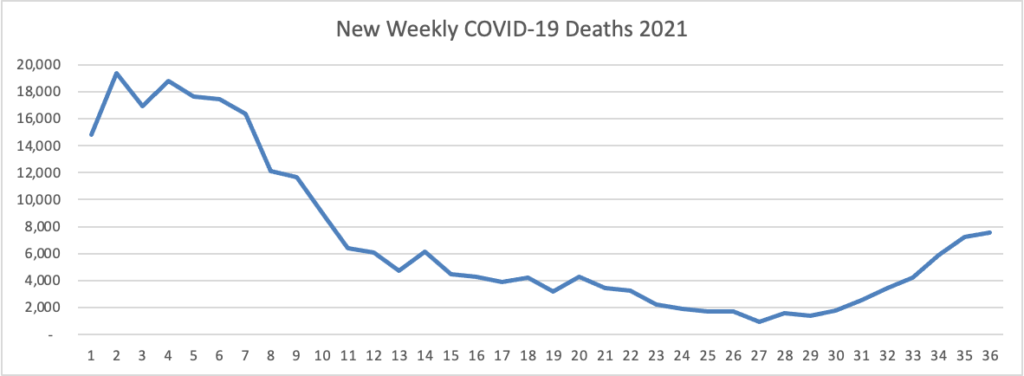
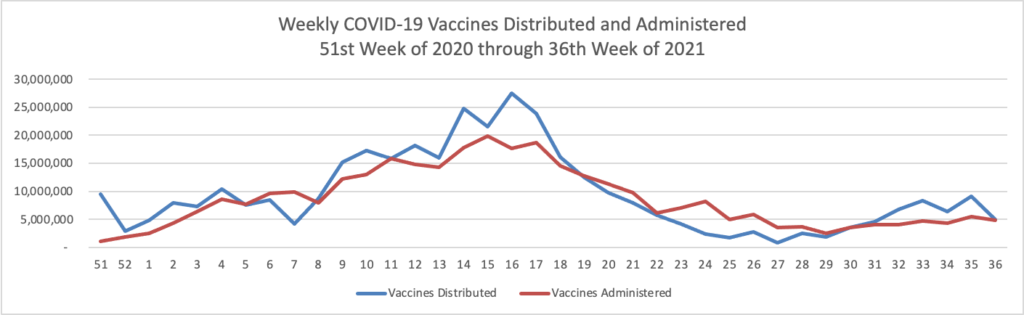
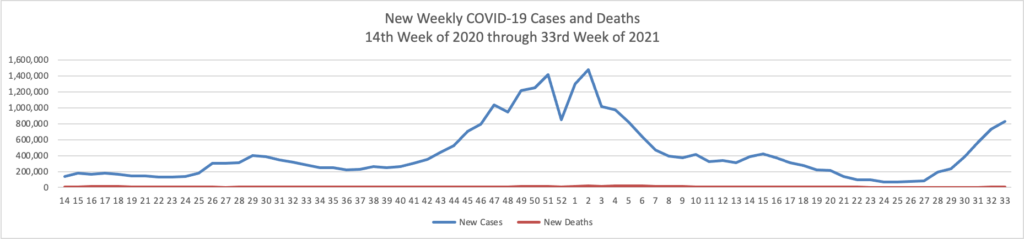
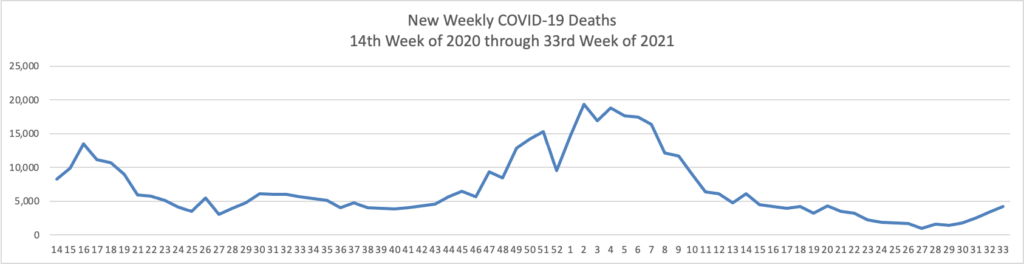
From the Delta variant front, the New York Times reports that “The federal government is expected to take a significant step this week toward offering booster doses to a much wider range of Americans as advisers to the Food and Drug Administration meet on Thursday and Friday [this week] to discuss recipients of the Johnson & Johnson and Moderna coronavirus vaccines.”
The Times also informs us that
Merck said on Monday that it had submitted an application to the Food and Drug Administration to authorize what would be the first antiviral pill to treat Covid.
Clearance for the drug, molnupiravir, would be a milestone in the fight against the coronavirus, experts said, because a convenient, relatively inexpensive treatment could reach many more high-risk people sick with Covid than the cumbersome antibody treatments currently being used.
The Biden administration is preparing for an authorization that could come within weeks; the pill would likely to be allocated to states, as was the case with the vaccines. States could then distribute the pills how they wish, such as through pharmacies or doctors’ practices, senior administration officials said.
If the pill wins authorization, tens of millions of Americans will most likely be eligible to take it if they get sick with Covid — many more than the supply could cover, at least initially. The federal government has placed an advance order for enough pills for 1.7 million Americans, at a price of about $700 per patient. That is about one-third the price that the government is paying for the monoclonal antibody treatments, which are generally given via intravenous infusion.
Fingers crossed on the pill.
From the hospital transparency front, Fierce Healthcare assesses a New York Times analysis of hospital pricing. Fierce Healthcare finds that cash prices can be lower than prices paid by insurers.
America’s Health Insurance Plans published a statement saying the attempt to look at the data “spotlights a lot of numbers with little context” and “often compares apples and oranges.”
Because of these complexities, the CMS rule does not help patients “shop for services” as intended, said Delphine O’Rourke, a healthcare attorney and partner at Goodwin Procter. “I, as a consumer, don’t know at the end of the day what I’m going to be responsible for,” she said.
To O’Rourke, it’s not surprising that at times, a hospital’s cash price is lower for a service. Since people paying cash price are generally a small segment of patients, she explained, and tend to be uninsured or undocumented, hospitals structure cash pay anticipating that it will be “challenging to collect,” O’Rourke said. (Earlier this year, the Wall Street Journal found that many times, patients who pay with cash are actually charged the highest price across hospitals.)
Be sure to listen to this week’s Econtalk episode during which Russ Roberts interviews Sam Quinones who wrote the FEHBlog’s favorite book of 2017, Dreamland. (While the book was published in 2015, the FEHBlog discovered it from a 2017 Econtalk episode.) This week Mr. Quinones discusses the tremendous impact of fentanyl on growing our opioid epidemic. He explains that dealers learned to lace fentanyl into non-addictive drugs like cocaine and meth thereby creating daily customers for them. Because Econtalk episodes last over an hour, you can find a transcript on the website. The FEHBlog has pre-ordered Mr. Quinones new book, the “Least of Us True Tales of America and Hope in the Time of Fentanyl and Meth,” which is available on Amazon for the Kindle at around nine dollars.