Weekend update

From Capitol Hill, The House of Representatives and the Senate will be in session for Committee business and floor voting this week. This is the last week that the House is in session before the August recess. The Senate has one more week of legislative work before it heads off on its State work period for the eighth month of the year.
The Wall Street Journal adds
Congress nears the close of a packed legislative session this week, aiming to pass legislation providing about $54 billion to boost U.S. semiconductor manufacturing while also juggling a raft of other bills ahead of the monthlong August recess.
Along with the bipartisan bill subsidizing chips, Democrats are hoping to salvage a piece of President Biden’s once-ambitious domestic agenda, looking to advance a measure aimed at lowering some drug and healthcare costs. The party is also weighing whether to hold votes related to social issues and guns that could help rally the party’s base. * * *
In the Senate, Democrats are awaiting guidance from the Senate’s parliamentarian over whether a measure aimed at lowering drug costs by giving Medicare the right to negotiate prices for a narrow set of drugs will comport with the Senate’s procedures for passing bills through a budget-related process known as reconciliation. Democrats in the 50-50 Senate are using the special process because it allows the passage of bills with only a simple majority, instead of the 60 votes required for most legislation. The procedure is only available until Sept. 30, the end of the fiscal year.
Mr. Hoyer said that if Senate Democrats are able to soon pass the drug-pricing bill, which would also extend Affordable Care Act subsidies, then the House would return from its August recess to clear the measure in time for insurance companies to factor in the subsidies when they set prices for their plans.
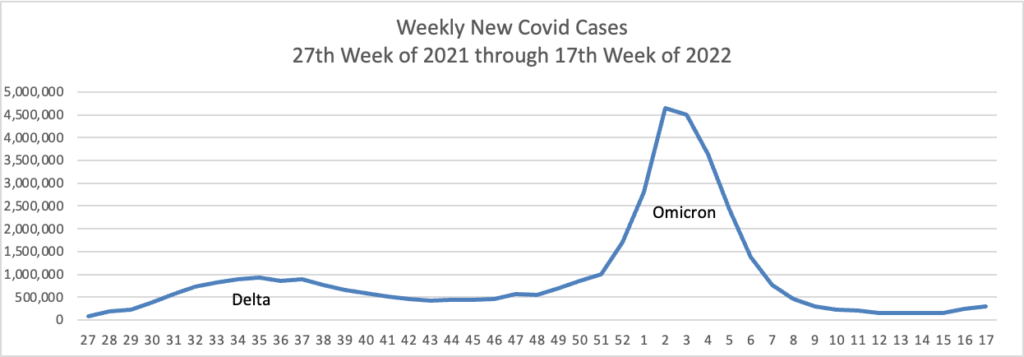
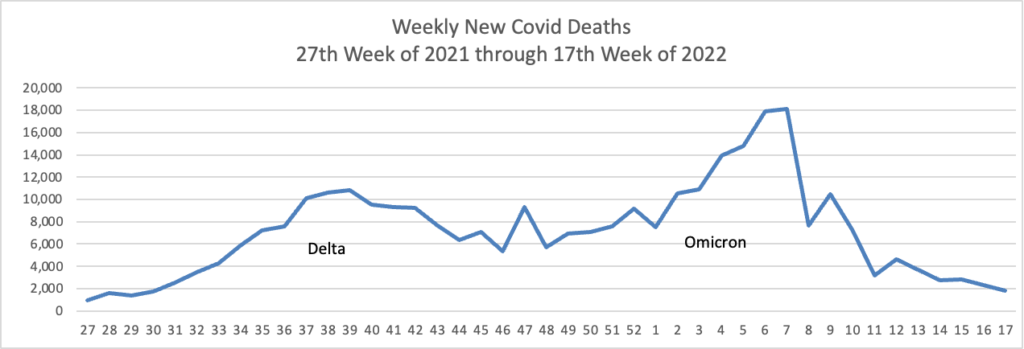
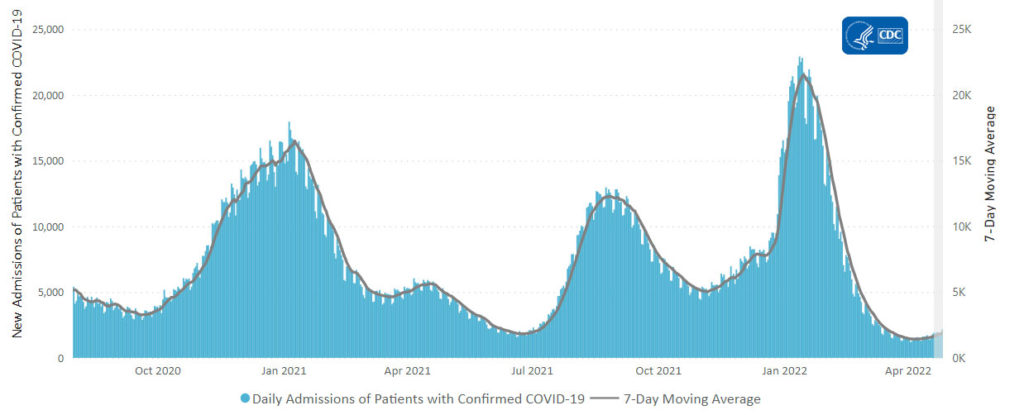
From the Omicron and siblings front, the New York Times informs us
People with coronavirus infections of the Omicron variant often have significantly different viral levels in their noses, throats and saliva, and testing just a single type of sample is likely to miss a large share of infections, according to two new papers, which analyzed Omicron infections over time in a small number of people.
The papers, which have not yet been published in scientific journals, suggest that coronavirus tests that analyze both nasal and throat swabs would pick up more Omicron infections than those that rely on just a nasal swab. Although these combined tests are common in other countries, including Britain, none are yet authorized in the United States.
“You could get a lot more bang for your buck if you use these mixed specimen types,” said Rustem Ismagilov, a chemist at the California Institute of Technology and the senior author of both papers. But in the United States, he said, “we are stuck with nobody doing it.”
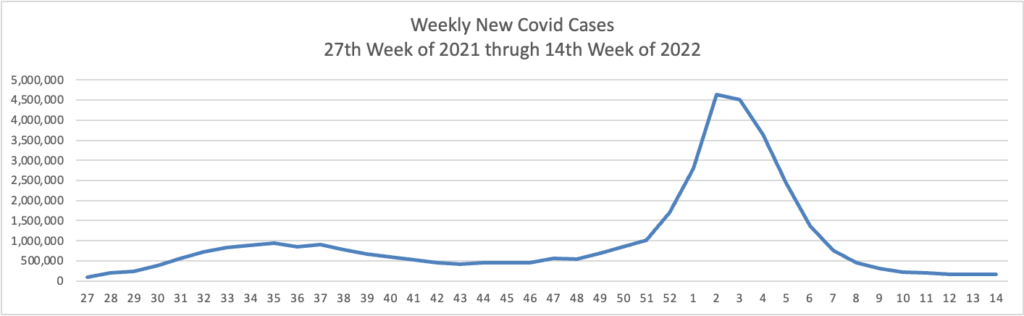
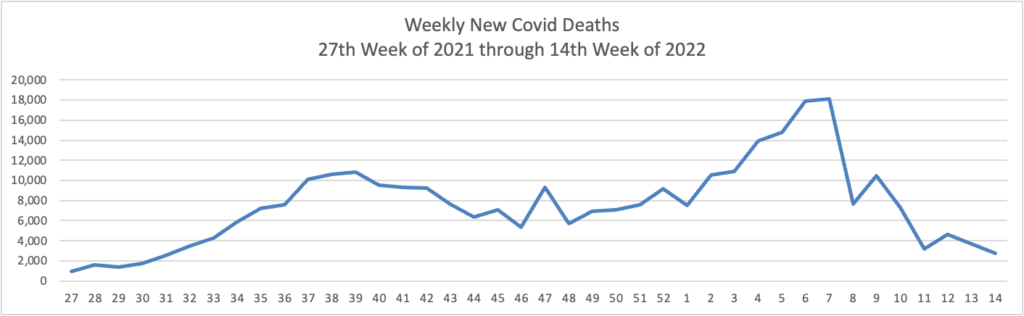
And yet the CDC has recorded nearly 90 million cases in our country.
From the unusual viruses front
STAT News reports
The World Health Organization on Saturday declared the unprecedented monkeypox outbreak that has spread around the world a public health emergency, a decision that will empower the agency to take additional measures to try to curb the virus’s spread.
In an unusual move, WHO Director-General Tedros Adhanom Ghebreyesus made the declaration even though a committee of experts he had convened to study the issue did not advise him to do so, having failed to reach a consensus. The same committee met just one month ago and declined to declare a public health emergency of international concern, or PHEIC [pronounced fake].
Secretary of Health and Human Services expressed his support for this decision.
Bloomberg Prognosis provides a quick take on monkeypox and its spread. Among other takes
The illness is usually mild and most patients will recover within a few weeks; treatment is mainly aimed at relieving symptoms. About 10-to-15% of cases have been hospitalized, mostly for pain and bacterial infections that can occur as a result of monkeypox lesions. The CDC says smallpox vaccine, antivirals, and vaccinia immune globulin can be used to treat monkeypox as well as control it.
The Wall Street Journal adds
Unusual for an emerging disease, there are already vaccines and treatments that can be used to counter monkeypox. That is because some governments have invested in developing defenses against the accidental or deliberate reintroduction of smallpox, a closely related but much more severe virus. Some countries hold these treatments and vaccines in national stockpiles, but they aren’t readily available everywhere.
In some places, including the U.S., U.K. and parts of Canada, broad groups of men who have sex with men are being offered vaccination in an effort to slow the spread, although vaccine supplies have so far been constrained. Public-health authorities also are working to raise awareness among men who have sex with men about the spread of monkeypox.
From the Medicare front, STAT News reports
The federal government is hashing out the details on a new type of rural hospital, and new developments suggest regulators want to make it an attractive option.
So-called Rural Emergency Hospitals (REH) will run emergency rooms, but won’t offer inpatient care. On top of bumped-up Medicare reimbursement, they’ll get facility payments north of $3 million annually, which is nothing to sneeze at for small hospitals. The new details were part of the government’s proposed Medicare payment rates for hospital outpatient services in 2023, released Friday [July 15].
For their part, hospitals aren’t yet sold on the idea.
From the Affordable Care Act front, we have Section 1557 news:
On Monday, July 25, 2022, [at 2:15 pm ET] the U.S. Department of Health and Human Services will announce a proposed rule to strengthen Section 1557 of the Affordable Care Act (ACA), which prohibits discrimination on the basis of race, color, national origin, sex, age, and disability in certain health programs and activities.
In regard to sex, which includes sexual orientation and gender identity, the proposed rule would solidify protections against discrimination consistent with the U.S. Supreme Court’s holding in Bostock v. Clayton County.
Strengthening this rule is a significant achievement for the Biden-Harris Administration and promotes gender and health equity and civil rights for communities of color, women, LGBTQI+ individuals, people with disabilities, persons with limited English proficiency (LEP), and seniors.
From the reports and studies department
- Fortune Well tells us “While two-thirds of Alzheimer’s cases are in women, an overwhelming majority don’t know they are at an increased risk for the disease, a new survey finds. A survey conducted by the Cleveland Clinic last month found that while 71% of women respondents saw a doctor for their health in the last year, 82% of them did not know that they are at increased risk for Alzheimer’s disease and 73% percent had not talked to their doctor about their brain health.”
- Healio points out
Early physician follow-up with a comprehensive transitional care strategy and effective chronic disease management after discharge was associated with reduced 90-day readmissions among patients with COPD and other complex conditions.
“This population-based retrospective cohort study found that early follow-up with a [primary care] physician or relevant specialist was associated with fewer readmissions for patients with [congestive heart failure] or COPD, fewer COPD-related readmissions for patients with COPD and lower mortality for patients with [congestive heart failure], all within 90 days of discharge, but that there was no demonstrable benefit at 30 days or for patients with [acute myocardial infarction],” Farah E. Saxena, MPH,researcher at the Canadian Partnership Against Cancer in Toronto, and colleagues wrote in JAMA Network Open.
- The CDC reports “Adults who receive diabetes education follow more recommended preventive care practices, such as getting regular physical activity.” Many FEHB plans offer diabetes education services.